J Korean Soc Magn Reson Med.
2013 Mar;17(1):8-18. 10.13104/jksmrm.2013.17.1.8.
Characteristic MRI Findings of Spinal Metastases from Various Primary Cancers: Retrospective Study of Pathologically-Confirmed Cases
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiological Science, Yonsei University College of Medicine, Seoul, Korea. hotsong@yuhs.ac
- KMID: 1426746
- DOI: http://doi.org/10.13104/jksmrm.2013.17.1.8
Abstract
- PURPOSE
The purpose of this study was to find and categorize the various magnetic resonance imaging (MRI) findings of spinal metastases that correlate with the type of primary cancer.
MATERIALS AND METHODS
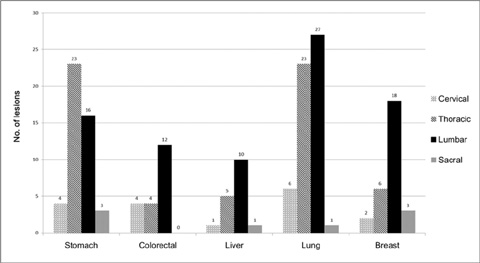
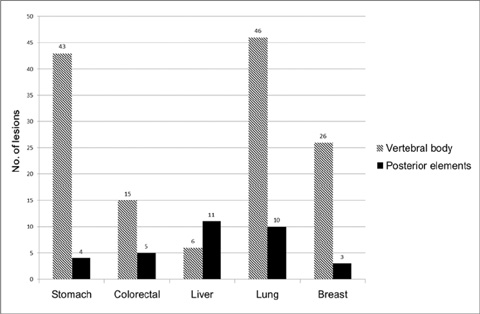
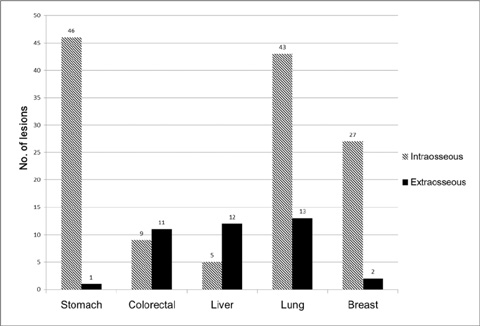
We retrospectively reviewed gadolinium-enhanced magnetic resonance images of 30 patients with 169 spinal metastatic lesions from lung cancer (n = 56), breast cancer (n = 29), colorectal cancer (n = 20), hepatocellular carcinoma (HCC) (n = 17), and stomach cancer (n = 47). The size, location, extent of invasion, signal intensity, margin, enhancement pattern, and osteoblastic or osteolytic characteristics of each metastatic tumor were analyzed.
RESULTS
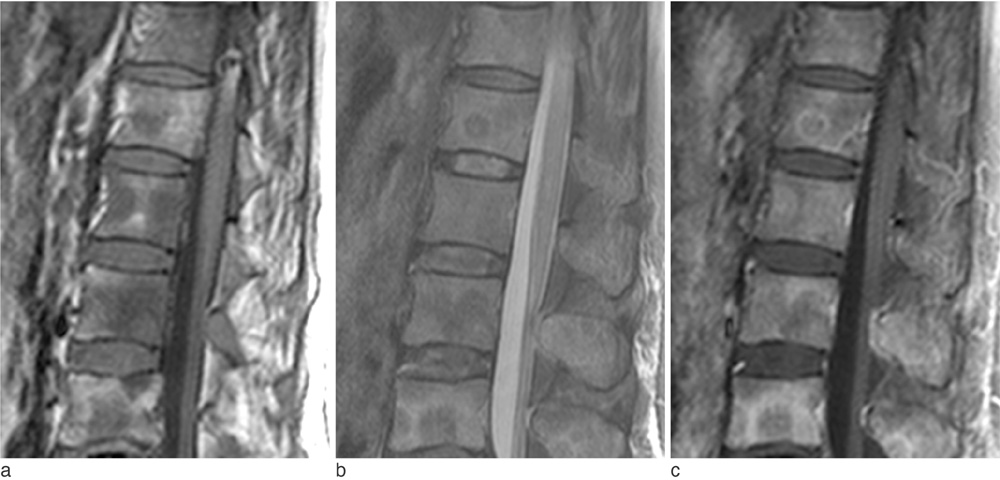
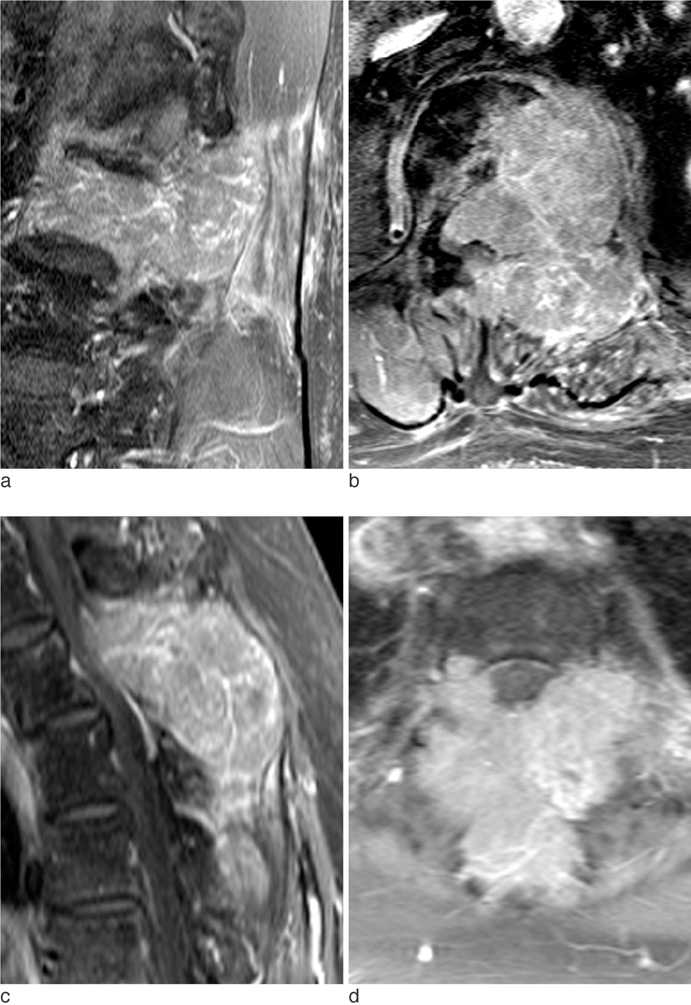
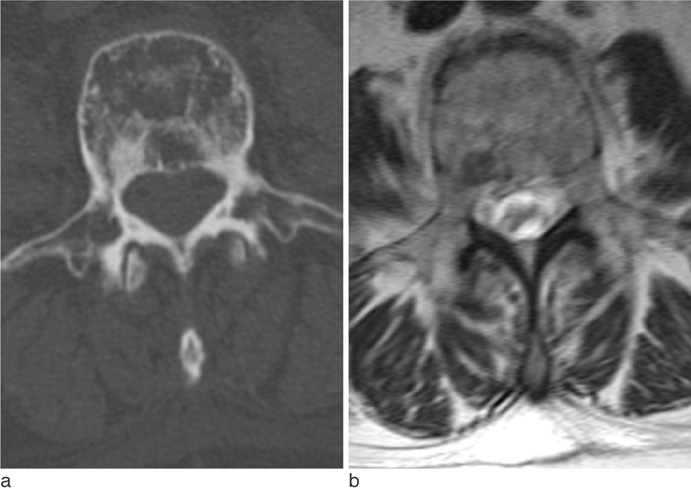
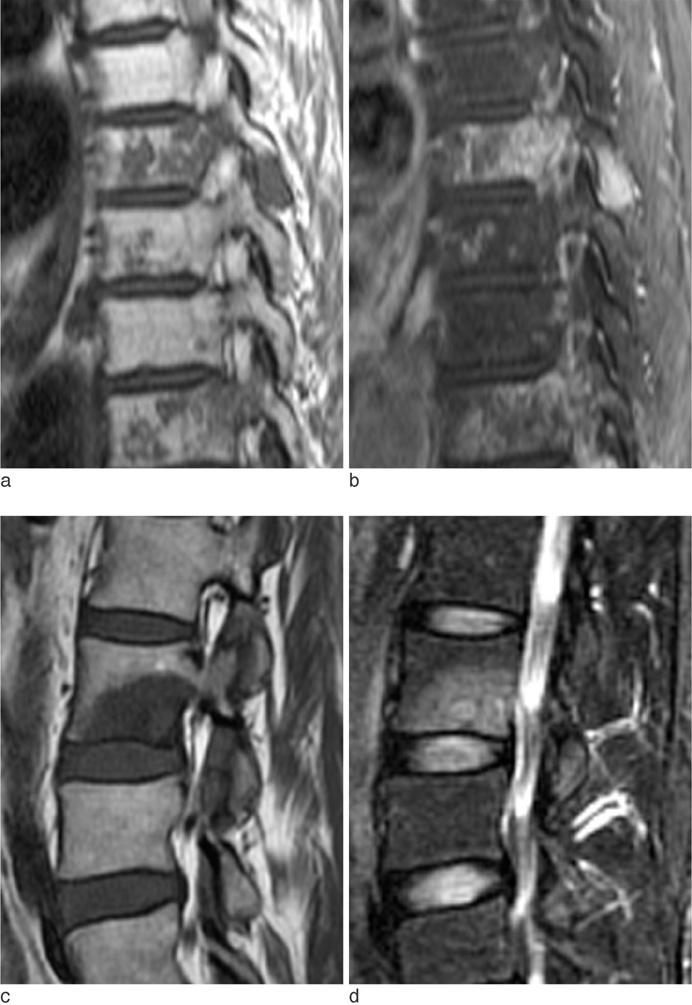
The metastatic lesions from HCC were larger than those from the other primary tumors (P < 0.05) except for colorectal cancer (P = 0.268). Well-defined metastatic tumor margins were more frequently seen in lung cancer and breast cancer (P < 0.01). All but HCC showed a tendency to invade the vertebral body rather than the posterior elements (P < 0.02). Colorectal cancer and HCC showed a tendency toward extraosseous invasion without statistical significance. HCC showed a characteristic enhancement pattern of 'worms-in-a-bag'. Rim enhancement with a sclerotic center was only seen in spinal metastases from stomach cancer.
CONCLUSION
Despite many overlapping imaging features, spinal metastases of various primary tumors display some characteristic MRI findings that can help identify the primary cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Boland PJ, Lane JM, Sundaresan N. Metastatic disease of the spine. Clin Orthop Relat Res. 1982; 169:95–102.2. Schiff D, O'Neill BP, Suman VJ. Spinal epidural metastasis as the initial manifestation of malignancy: clinical features and diagnostic approach. Neurology. 1997; 49:452–456.3. Berrettoni BA, Carter JR. Mechanisms of cancer metastasis to bone. J Bone Joint Surg Am. 1986; 68:308–312.4. Guillevin R, Vallee JN, Lafitte F, Menuel C, Duverneuil NM, Chiras J. Spine metastasis imaging: review of the literature. J Neuroradiol. 2007; 34:311–321.5. Daugaard G. Unknown primary tumours. Cancer Treat Rev. 1994; 20:119–147.6. Abbruzzese JL, Abbruzzese MC, Hess KR, Raber MN, Lenzi R, Frost P. Unknown primary carcinoma: natural history and prognostic factors in 657 consecutive patients. J Clin Oncol. 1994; 12:1272–1280.7. Katagiri H, Takahashi M, Inagaki J, Sugiura H, Ito S, Iwata H. Determining the site of the primary cancer in patients with skeletal metastasis of unknown origin: a retrospective study. Cancer. 1999; 86:533–537.8. Brihaye J, Ectors P, Lemort M, Van Houtte P. The management of spinal epidural metastases. Adv Tech Stand Neurosurg. 1988; 16:121–176.9. Ghanem N, Uhl M, Brink I, et al. Diagnostic value of MRI in comparison to scintigraphy, PET, MS-CT and PET/CT for the detection of metastases of bone. Eur J Radiol. 2005; 55:41–55.10. Chiewvit P, Danchaivijitr N, Sirivitmaitrie K, Chiewvit S, Thephamongkhol K. Does magnetic resonance imaging give value-added than bone scintigraphy in the detection of vertebral metastasis? J Med Assoc Thai. 2009; 92:818–829.11. Beltran J, Noto AM, Chakeres DW, Christoforidis AJ. Tumors of the osseous spine: staging with MR imaging versus CT. Radiology. 1987; 162:565–569.12. Keogh C, Bergin D, Brennan D, Eustace S. MR imaging of bone tumors of the cervical spine. Magn Reson Imaging Clin N Am. 2000; 8:513–528.13. Ross JS, Moore KR, Borg B, Crim J, Shah LM. Diagnostic imaging: Spine. 2nd ed. Salt Lake City: Amirsys;2010.14. Sugimura K, Kajitani A, Okizuka H, Sugihara M, Mizutani M, Ishida T. Assessing response to therapy of spinal metastases with gadolinium-enhanced MR imaging. J Magn Reson Imaging. 1991; 1:481–484.15. Sneag DB, Krajewski K, Giardino A, et al. Extrahepatic spread of hepatocellular carcinoma: spectrum of imaging findings. AJR Am J Roentgenol. 2011; 197:W658–W664.16. Katyal S, Oliver JH 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000; 216:698–703.17. Seo HJ, Choi YJ, Kim HJ, et al. Evaluation of bone metastasis from hepatocellular carcinoma using 18F-FDG PET/CT and 99mTc-HDP bone scintigraphy: characteristics of soft tissue formation. Nucl Med Mol Imaging. 2011; 45:203–211.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Prognoses according to Breast MRI Results in Patients with Axillary Lymph Node Metastases from an Unknown Primary Origin
- MR Myelography
- Spinal Presentation of Spontaneous Intracranial Hypotension
- MR findings of spinal epidural mass
- Can Initial 18F-FDG PET-CT Imaging Give Information onMetastasis in Patients with Primary Renal Cell Carcinoma?