J Korean Soc Surg Hand.
2012 Dec;17(4):173-182. 10.12790/jkssh.2012.17.4.173.
Absorbable Barbed Suture for the Repair of Tendo Calcaneus in Rabbits
- Affiliations
-
- 1Department of Plastic and Reconstructive Surgery, Seoul National University College of Medicine, Seoul, Korea. stk59@snu.ac.kr
- KMID: 1425260
- DOI: http://doi.org/10.12790/jkssh.2012.17.4.173
Abstract
- PURPOSE
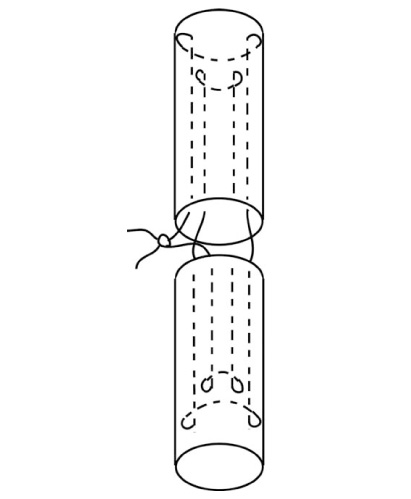
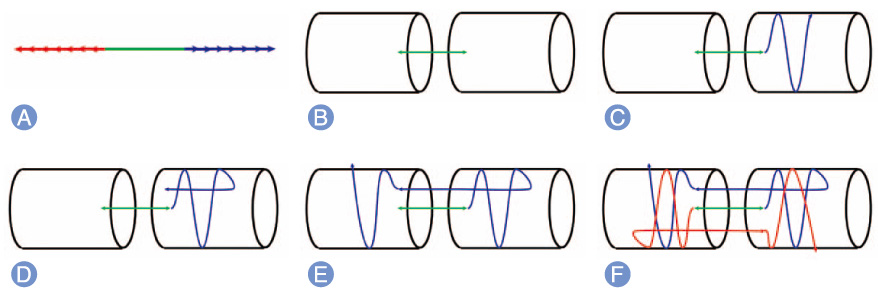
A barbed suture used in flexor tenorrhaphy can maintain prolonged strength despite absorption of the suture material and allows knotless repair with tendon-barb adherence along the suture's entire length. The purpose of this study was to evaluate the strength of the tendon and its histologic analysis after tenorrhaphy using barbed sutures.
METHODS
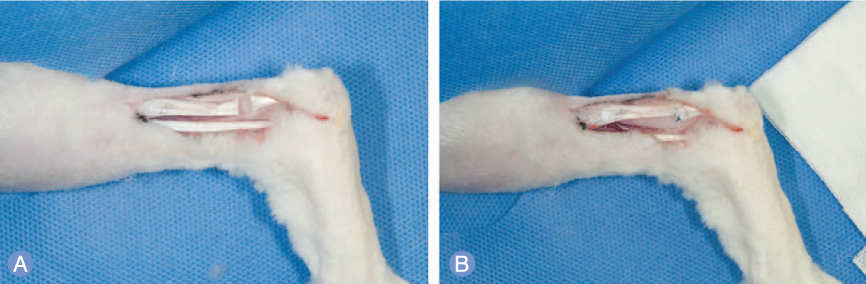
Forty-two New Zealand rabbits were used in this study and were divided into experimental and control groups. In the experimental group, knotless repair of the tendons was performed using absorbable barbed sutures. In the control group, a 4-stranded double-modified Kessler tenorrhaphy was performed using non-absorbable monofilament sutures. The force to failure for each tendon was measured immediately after tendon repair and at 1 week, 4 weeks, and 8 weeks after the repair. Microscopic analysis of the tendons was performed at 1 week, 4 weeks, and 8 weeks after their repair.
RESULTS
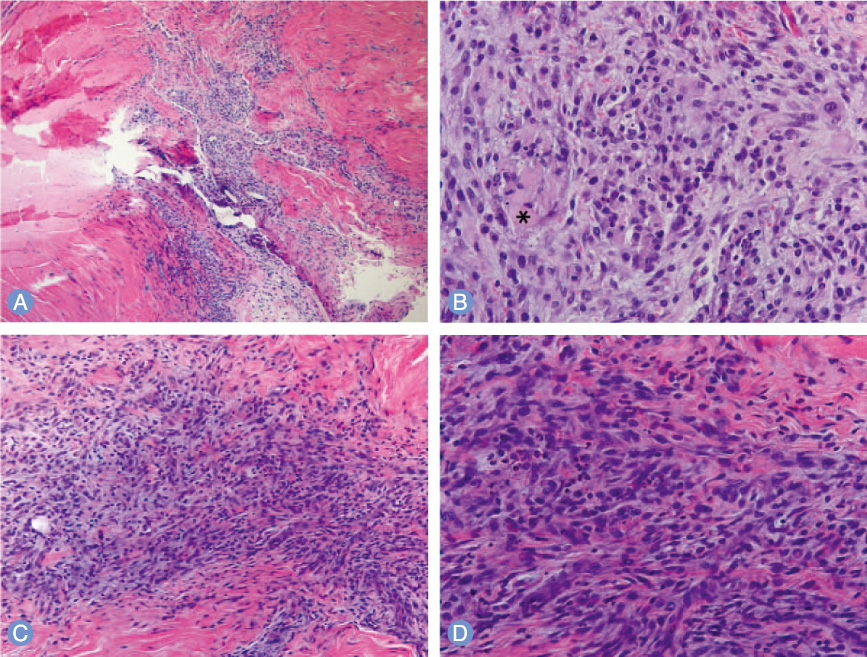
Eight weeks after tendon repair, the force to failure value of the rabbits in the experimental group (144.02+/-10.21 N) was significantly higher than that of the rabbits in the control group (125.26+/-8.75 N) (p=0.032). The difference in the value during the periods was not statistically significant. Histologic findings showed increased foreign body reaction in the tendons of the experimental group and sustained inflammation in those of the control group.
CONCLUSION
With respect to load to failure and the degree of inflammation, the use of absorbable barbed suture resulted in better tendon repair than the conventional non-absorbable suture.
Keyword
Figure
Reference
-
1. Strickland JW. Flexor tendon surgery. Part 1: primary flexor tendon repair. J Hand Surg Br. 1989. 14:261–272.2. Silfverskiold KL, May EJ. Flexor tendon repair in zone II with a new suture technique and an early mobilization program combining passive and active flexion. J Hand Surg Am. 1994. 19:53–60.3. Thurman RT, Trumble TE, Hanel DP, Tencer AF, Kiser PK. Two-, four-, and six-strand zone II flexor tendon repairs: an in situ biomechanical comparison using a cadaver model. J Hand Surg Am. 1998. 23:261–265.4. Zaruby J, Gingras K, Taylor J, Maul D. An in vivo comparison of barbed suture devices and conventional monofilament sutures for cosmetic skin closure: biomechanical wound strength and histology. Aesthet Surg J. 2011. 31:232–240.
Article5. Parikh PM, Davison SP, Higgins JP. Barbed suture tenorrhaphy: an ex vivo biomechanical analysis. Plast Reconstr Surg. 2009. 124:1551–1558.
Article6. Goodman HJ, Choueka J. Biomechanics of the flexor tendons. Hand Clin. 2005. 21:129–149.
Article7. Schuind F, Garcia-Elias M, Cooney WP 3rd, An KN. Flexor tendon forces: in vivo measurements. J Hand Surg Am. 1992. 17:291–298.
Article8. Lawrence TM, Davis TR. A biomechanical analysis of suture materials and their influence on a four-strand flexor tendon repair. J Hand Surg Am. 2005. 30:836–841.
Article9. Villa MT, White LE, Alam M, Yoo SS, Walton RL. Barbed sutures: a review of the literature. Plast Reconstr Surg. 2008. 121:102e–108e.
Article10. Rashid RM, Sartori M, White LE, Villa MT, Yoo SS, Alam M. Breaking strength of barbed polypropylene sutures: rater-blinded, controlled comparison with nonbarbed sutures of various calibers. Arch Dermatol. 2007. 143:869–872.11. Mashadi ZB, Amis AA. Variation of holding strength of synthetic absorbable flexor tendon sutures with time. J Hand Surg Br. 1992. 17:278–281.
Article12. Enwemeka CS. Functional loading augments the initial tensile strength and energy absorption capacity of regenerating rabbit Achilles tendons. Am J Phys Med Rehabil. 1992. 71:31–38.
Article13. Boyer MI. Flexor tendon biology. Hand Clin. 2005. 21:159–166.
Article14. Strickland JW. Flexor Tendon Injuries: I. Foundations of Treatment. J Am Acad Orthop Surg. 1995. 3:44–54.
Article15. Yildirim Y, Kara H, Cabukoglu C, Esemenli T. Suture holding capacity of the Achilles tendon during the healing period: an in vivo experimental study in rabbits. Foot Ankle Int. 2006. 27:121–124.
Article16. Strickland JW. Development of flexor tendon surgery: twenty-five years of progress. J Hand Surg Am. 2000. 25:214–235.
Article17. Pruitt DL, Manske PR, Fink B. Cyclic stress analysis of flexor tendon repair. J Hand Surg Am. 1991. 16:701–707.
Article18. Williams RJ, Amis AA. A new type of flexor tendon repair. Biomechanical evaluation by cyclic loading, ultimate strength and assessment of pulley friction in vitro. J Hand Surg Br. 1995. 20:578–583.19. Strickland JW. The scientific basis for advances in flexor tendon surgery. J Hand Ther. 2005. 18:94–110.
Article20. Amiel D, Gelberman R, Harwood F, Siegel D. Fibronectin in healing flexor tendons subjected to immobilization or early controlled passive motion. Matrix. 1991. 11:184–189.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reversed Tendon Sheath Wrapping on Indirect Rupture of Tendo Calcaneus
- Postoperative mechanical small bowel obstruction induced by V-Loc barbed absorbable suture after laparoscopic distal gastrectomy
- Surgical and Clinical Outcomes Associated With the Use of Barbed Sutures and Self-Adhering Mesh System and Polymeric Glue for Wound Closure in Multilevel or Revision Spinal Surgery: A Matched Cohort Comparative Study With Conventional Wound Closure Procedure
- Adhesion Power and Histopathologic Findings After Muscle-scleral Tuck of Extraocular Muscles with Absorbable Suture Material in Rabbits
- Barbed sutures versus conventional tenorrhaphy in flexor tendon repair: An ex vivo biomechanical analysis