Korean J Radiol.
2012 Aug;13(4):425-433. 10.3348/kjr.2012.13.4.425.
Small Submucosal Tumors of the Stomach: Differentiation of Gastric Schwannoma from Gastrointestinal Stromal Tumor with CT
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea. dichoi@skku.edu
- 2Department of Pathology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea.
- 3Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea.
- 4Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea.
- 5Department of Radiology, Kangwon National University College of Medicine, Chuncheon 200-701, Korea.
- KMID: 1383854
- DOI: http://doi.org/10.3348/kjr.2012.13.4.425
Abstract
OBJECTIVE
To identify the CT features that help differentiate gastric schwannomas (GS) from small (5 cm or smaller) gastrointestinal stromal tumors (GIST) and to assess the growth rates of both tumors.
MATERIALS AND METHODS
We included 16 small GSs and 56 GISTs located in the stomach. We evaluated the CT features including size, contour, surface pattern, margins, growth pattern, pattern and degree of contrast enhancement, and the presence of intralesional low attenuation area, hemorrhage, calcification, surface dimpling, fistula, perilesional lymph nodes (LNs), invasion to other organs, metastasis, ascites, and peritoneal seeding. We also estimated the tumor volume doubling time.
RESULTS
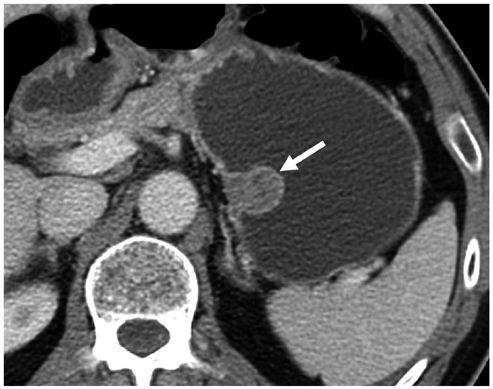
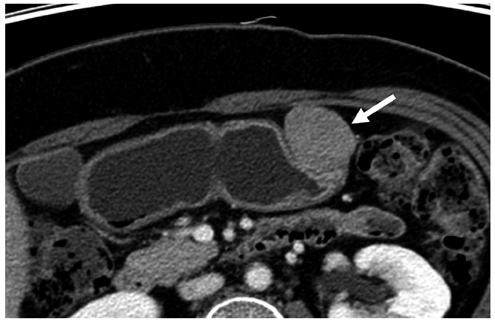
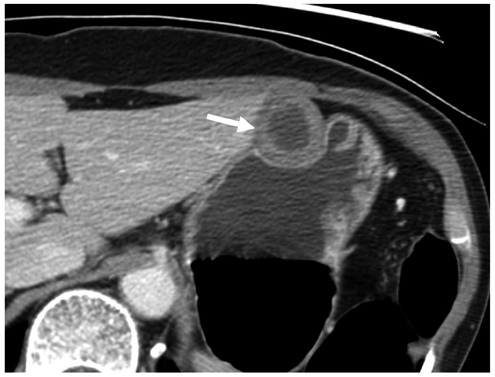
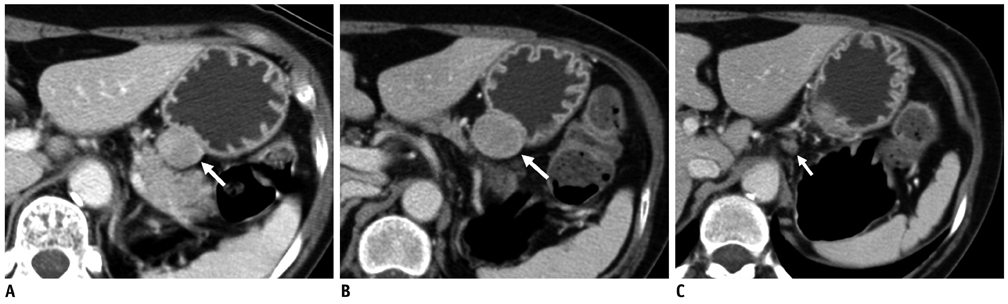
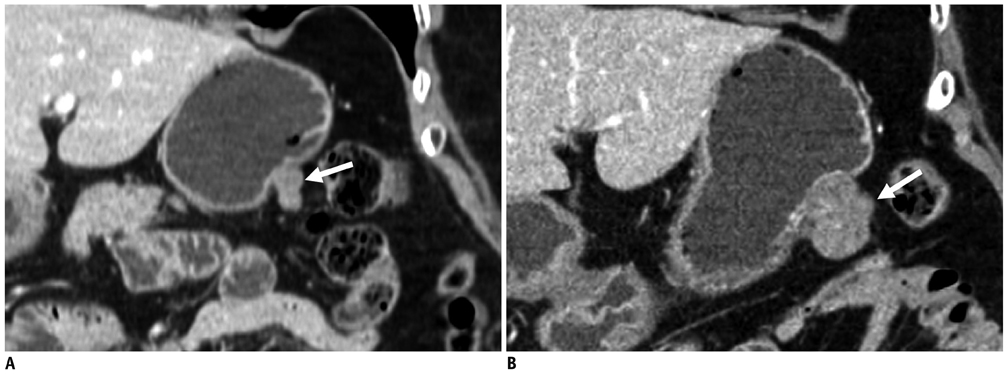
Compared with GISTs, GSs more frequently demonstrated a homogeneous enhancement pattern, exophytic or mixed growth pattern, and the presence of perilesional LNs (each p < 0.05). The intralesional low attenuation area was more common in GISTs than GSs (p < 0.05). Multivariate analyses indicated that a homogeneous enhancement pattern, exophytic or mixed growth pattern, and the presence of perilesional LNs were statistically significant (p < 0.05). Tumor volume doubling times for GSs (mean, 1685.4 days) were significantly longer than that of GISTs (mean, 377.6 days) (p = 0.004).
CONCLUSION
Although small GSs and GISTs show similar imaging findings, GSs more frequently show an exophytic or mixed growth pattern, homogeneous enhancement pattern, perilesional LNs and grow slower than GISTs.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Contrast Media/diagnostic use
Diagnosis, Differential
Endoscopy, Gastrointestinal
Female
Gastrointestinal Stromal Tumors/pathology/*radiography
Humans
Iohexol/analogs & derivatives/diagnostic use
Logistic Models
Male
Middle Aged
Neurilemmoma/pathology/*radiography
Retrospective Studies
Statistics, Nonparametric
Stomach Neoplasms/pathology/*radiography
Tomography, X-Ray Computed/*methods
Figure
Cited by 2 articles
-
Surgical Treatment of Gastric Gastrointestinal Stromal Tumor
Seong-Ho Kong, Han-Kwang Yang
J Gastric Cancer. 2013;13(1):3-18. doi: 10.5230/jgc.2013.13.1.3.Endoscopic Ultrasonographic Characteristics of Gastric Schwannoma Distinguished from Gastrointestinal Stromal Tumor
Hyung-Chul Park, Dong-Jun Son, Hyung-Hoon Oh, Chan-Young Oak, Mi-Young Kim, Cho-Yun Chung, Dae-Seong Myung, Jong-Sun Jong-Sun, Sung-Bum Cho, Wan-Sik Lee, Young-Eun Joo
Korean J Gastroenterol. 2015;65(1):21-26. doi: 10.4166/kjg.2015.65.1.21.
Reference
-
1. Day DD, Jass JR, Price AB, Shepherd NA, Sloan JM, Talbot IC, et al. Morson & dawson's gastrointestinal pathology. 2003. Massachusetts: Blackwell Science Ltd..2. Nishida T, Hirota S. Biological and clinical review of stromal tumors in the gastrointestinal tract. Histol Histopathol. 2000. 15:1293–1301.3. Okai T, Minamoto T, Ohtsubo K, Minato H, Kurumaya H, Oda Y, et al. Endosonographic evaluation of c-kit-positive gastrointestinal stromal tumor. Abdom Imaging. 2003. 28:301–307.4. Hong HS, Ha HK, Won HJ, Byun JH, Shin YM, Kim AY, et al. Gastric schwannomas: radiological features with endoscopic and pathological correlation. Clin Radiol. 2008. 63:536–542.5. Jung MK, Jeon SW, Cho CM, Tak WY, Kweon YO, Kim SK, et al. Gastric schwannomas: endosonographic characteristics. Abdom Imaging. 2008. 33:388–390.6. Levy AD, Quiles AM, Miettinen M, Sobin LH. Gastrointestinal schwannomas: CT features with clinicopathologic correlation. AJR Am J Roentgenol. 2005. 184:797–802.7. Levy AD, Remotti HE, Thompson WM, Sobin LH, Miettinen M. Gastrointestinal stromal tumors: radiologic features with pathologic correlation. Radiographics. 2003. 23:283–304. 456quiz 532.8. Ghanem N, Altehoefer C, Furtwängler A, Winterer J, Schäfer O, Springer O, et al. Computed tomography in gastrointestinal stromal tumors. Eur Radiol. 2003. 13:1669–1678.9. Kim JY, Lee JM, Kim KW, Park HS, Choi JY, Kim SH, et al. Ectopic pancreas: CT findings with emphasis on differentiation from small gastrointestinal stromal tumor and leiomyoma. Radiology. 2009. 252:92–100.10. Kim JK, Won JH, Cho YK, Kim MW, Joo HJ, Suh JH. Glomus tumor of the stomach: CT findings. Abdom Imaging. 2001. 26:303–305.11. Kang JH, Lim JS, Kim JH, Hyung WJ, Chung YE, Choi JY, et al. Role of EUS and MDCT in the diagnosis of gastric submucosal tumors according to the revised pathologic concept of gastrointestinal stromal tumors. Eur Radiol. 2009. 19:924–934.12. Kim HC, Lee JM, Choi SH, Han H, Kim SS, Lee SH, et al. Cystic changes in intraabdominal extrahepatic metastases from gastrointestinal stromal tumors treated with imatinib. Korean J Radiol. 2004. 5:157–163.13. Breiman RS, Beck JW, Korobkin M, Glenny R, Akwari OE, Heaston DK, et al. Volume determinations using computed tomography. AJR Am J Roentgenol. 1982. 138:329–333.14. Schwartz M. A biomathematical approach to clinical tumor growth. Cancer. 1961. 14:1272–1294.15. Tateishi U, Hasegawa T, Satake M, Moriyama N. Gastrointestinal stromal tumor. Correlation of computed tomography findings with tumor grade and mortality. J Comput Assist Tomogr. 2003. 27:792–779.16. Kim HC, Lee JM, Kim KW, Park SH, Kim SH, Lee JY, et al. Gastrointestinal stromal tumors of the stomach: CT findings and prediction of malignancy. AJR Am J Roentgenol. 2004. 183:893–898.17. Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, et al. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009. 125:666–673.18. Trupiano JK, Stewart RE, Misick C, Appelman HD, Goldblum JR. Gastric stromal tumors: a clinicopathologic study of 77 cases with correlation of features with nonaggressive and aggressive clinical behaviors. Am J Surg Pathol. 2002. 26:705–714.19. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002. 33:459–465.20. Miettinen M, El-Rifai W, H L Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002. 33:478–483.21. Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005. 16:566–578.22. Franquemont DW. Differentiation and risk assessment of gastrointestinal stromal tumors. Am J Clin Pathol. 1995. 103:41–47.23. Hornick JL, Fletcher CD. The role of KIT in the management of patients with gastrointestinal stromal tumors. Hum Pathol. 2007. 38:679–687.24. Keun Park C, Lee EJ, Kim M, Lim HY, Choi DI, Noh JH, et al. Prognostic stratification of high-risk gastrointestinal stromal tumors in the era of targeted therapy. Ann Surg. 2008. 247:1011–1018.25. Binstock AJ, Johnson CD, Stephens DH, Lloyd RV, Fletcher JG. Carcinoid tumors of the stomach: a clinical and radiographic study. AJR Am J Roentgenol. 2001. 176:947–951.26. Yan SL, Yeh YH, Chen CH, Yang CC, Kuo CL, Wu HS. Gastric glomus tumor: a hypervascular submucosal tumor on power Doppler endosonography. J Clin Ultrasound. 2007. 35:164–168.27. Daimaru Y, Kido H, Hashimoto H, Enjoji M. Benign schwannoma of the gastrointestinal tract: a clinicopathologic and immunohistochemical study. Hum Pathol. 1988. 19:257–264.28. Sarlomo-Rikala M, Miettinen M. Gastric schwannoma--a clinicopathological analysis of six cases. Histopathology. 1995. 27:355–360.29. Akahoshi K, Sumida Y, Matsui N, Oya M, Akinaga R, Kubokawa M, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007. 13:2077–2082.30. Ando N, Goto H, Niwa Y, Hirooka Y, Ohmiya N, Nagasaka T, et al. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002. 55:37–43.31. Kumar AJ, Kuhajda FP, Martinez CR, Fishman EK, Jezic DV, Siegelman SS. Computed tomography of extracranial nerve sheath tumors with pathological correlation. J Comput Assist Tomogr. 1983. 7:857–865.32. Cohen LM, Schwartz AM, Rockoff SD. Benign schwannomas: pathologic basis for CT inhomogeneities. AJR Am J Roentgenol. 1986. 147:141–143.33. Rha SE, Byun JY, Jung SE, Chun HJ, Lee HG, Lee JM. Neurogenic tumors in the abdomen: tumor types and imaging characteristics. Radiographics. 2003. 23:29–43.34. Kwon MS, Lee SS, Ahn GH. Schwannomas of the gastrointestinal tract: clinicopathological features of 12 cases including a case of esophageal tumor compared with those of gastrointestinal stromal tumors and leiomyomas of the gastrointestinal tract. Pathol Res Pract. 2002. 198:605–613.35. Prévot S, Bienvenu L, Vaillant JC, de Saint-Maur PP. Benign schwannoma of the digestive tract: a clinicopathologic and immunohistochemical study of five cases, including a case of esophageal tumor. Am J Surg Pathol. 1999. 23:431–436.