Rapid Diagnosis of Tuberculosis and Multidrug Resistance Using a MGIT 960 System
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 2Korean Institute of Tuberculosis, Osong, Korea.
- 3Department of Laboratory Medicine & Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. micro.lee@samsung.com
- KMID: 1380085
- DOI: http://doi.org/10.3343/alm.2012.32.4.264
Abstract
- BACKGROUND
The purpose of this study was to compare the turnaround time for liquid culturing and primary anti-tuberculous drug susceptibility testing (DST) performed using the mycobacteria growth indicator tube (MGIT) 960 system (Becton Dickinson, USA) with that for conventional culturing and DST (by the absolute concentration method) performed using solid culture medium and to determine the concordance rates of DST results obtained using these 2 methods.
METHODS
In this retrospective study, we compared the turnaround times from receiving the request for mycobacterial culture to reporting the DST results before and after the introduction of the MGIT 960 system. Further, we determined the concordance between DST results for isoniazid and rifampin for Mycobacterium tuberculosis isolates obtained using the MGIT 960 system and the absolute concentration method, which was conducted at the Korean Institute of Tuberculosis.
RESULTS
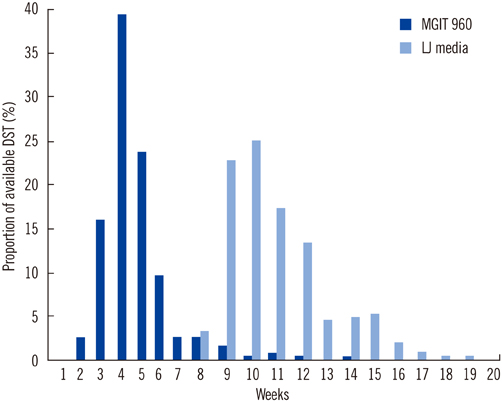
The overall turnaround time for mycobacterial culturing and DST was 27 days for liquid culturing and DST using the MGIT 960 system versus approximately 70 days for culturing on solid medium and DST with the absolute concentration method (P<0.001). There was a good concordance between findings of DST obtained with the 2 methods (97.2%, kappa coefficient=0.855 for rifampin; and 95.6%, kappa coefficient=0.864 for isoniazid), for 1,083 clinical isolates.
CONCLUSIONS
The automated MGIT 960 system for culturing and DST of M. tuberculosis was successfully introduced in a hospital laboratory setting in Korea with significant shortening of the turnaround time.
Keyword
MeSH Terms
-
Antitubercular Agents/*pharmacology
Automation
*Drug Resistance, Multiple, Bacterial/drug effects
Humans
Isoniazid/pharmacology
*Microbial Sensitivity Tests/instrumentation/methods
Mycobacterium tuberculosis/*drug effects/growth & development/isolation & purification
Retrospective Studies
Rifampin/pharmacology
Time Factors
Tuberculosis/*diagnosis
Figure
Cited by 4 articles
-
Diagnosis of pulmonary tuberculosis
Byung Woo Jhun, Hee Jae Huh, Won-Jung Koh
J Korean Med Assoc. 2019;62(1):18-24. doi: 10.5124/jkma.2019.62.1.18.Early Detection of Mycobacteria Using a Novel Hydrogel Culture Method
Mi Hee Jang, Shine Young Kim, Chang-Ki Kim, Sang-Hyun Hwang, Byung Kyu Park, Sung Soo Kim, Eun Yup Lee, Chulhun L. Chang
Ann Lab Med. 2014;34(1):26-30. doi: 10.3343/alm.2014.34.1.26.Diagnosis and treatment of multidrug-resistant tuberculosis
Jong Geol Jang, Jin Hong Chung
Yeungnam Univ J Med. 2020;37(4):277-285. doi: 10.12701/yujm.2020.00626.Clinical Course of Patients With Mediastinal Lymph Node Tuberculosis and Risk Factors for Paradoxical Responses
Junsu Choe, Areum Han, Sun Hye Shin, Kyungjong Lee, Sang-Won Um, Hojoong Kim, Tae Yeul Kim, Hee Jae Huh, Yoon-La Choi, Joungho Han, Byeong-Ho Jeong
J Korean Med Sci. 2023;38(47):e348. doi: 10.3346/jkms.2023.38.e348.
Reference
-
1. Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009. 9:153–161.
Article2. Johnston JC, Shahidi NC, Sadatsafavi M, Fitzgerald JM. Treatment outcomes of multidrug-resistant tuberculosis: a systematic review and meta-analysis. PLoS One. 2009. 4:e6914.
Article3. Kim HR, Hwang SS, Kim HJ, Lee SM, Yoo CG, Kim YW, et al. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin Infect Dis. 2007. 45:1290–1295.
Article4. Kwon YS, Kim YH, Suh GY, Chung MP, Kim H, Kwon OJ, et al. Treatment outcomes for HIV-uninfected patients with multidrug-resistant and extensively drug-resistant tuberculosis. Clin Infect Dis. 2008. 47:496–502.
Article5. Kim DH, Kim HJ, Park SK, Kong SJ, Kim YS, Kim TH, et al. Treatment outcomes and long-term survival in patients with extensively drug-resistant tuberculosis. Am J Respir Crit Care Med. 2008. 178:1075–1082.
Article6. Jeon DS, Shin DO, Park SK, Seo JE, Seo HS, Cho YS, et al. Treatment outcome and mortality among patients with multidrug-resistant tuberculosis in tuberculosis hospitals of the public sector. J Korean Med Sci. 2011. 26:33–41.
Article7. Van Deun A, Martin A, Palomino JC. Diagnosis of drug-resistant tuberculosis: reliability and rapidity of detection. Int J Tuberc Lung Dis. 2010. 14:131–140.8. O'Grady J, Maeurer M, Mwaba P, Kapata N, Bates M, Hoelscher M, et al. New and improved diagnostics for detection of drug-resistant pulmonary tuberculosis. Curr Opin Pulm Med. 2011. 17:134–141.9. Kim SJ. Drug-susceptibility testing in tuberculosis: methods and reliability of results. Eur Respir J. 2005. 25:564–569.
Article10. Wright A, Zignol M, Van Deun A, Falzon D, Gerdes SR, Feldman K, et al. Epidemiology of antituberculosis drug resistance 2002-07: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet. 2009. 373:1861–1873.
Article11. Schaberg T, Reichert B, Schulin T, Lode H, Mauch H. Rapid drug susceptibility testing of Mycobacterium tuberculosis using conventional solid media. Eur Respir J. 1995. 8:1688–1693.12. Muralidhar S, Srivastava L. Evaluation of three methods to determine the antimicrobial susceptibility of Mycobacterium tuberculosis. Indian J Med Res. 2004. 120:463–467.13. World Health Organization (WHO). Use of liquid TB culture and drug susceptibility testing (DST) in low- and medium-income settings. Summary report of the Expert Group Meeting on the Use of Liquid Culture Media. 2007. Geneva, Switzerland: WHO.14. Bai GH, Park YK, Choi YW, Bai JI, Kim HJ, Chang CL, et al. Trend of anti-tuberculosis drug resistance in Korea, 1994-2004. Int J Tuberc Lung Dis. 2007. 11:571–576.15. Choi JC, Lim SY, Suh GY, Chung MP, Kim H, Kwon OJ, et al. Drug Resistance Rates of Mycobacterium tuberculosis at a Private Referral Center in Korea. J Korean Med Sci. 2007. 22:677–681.
Article16. Bae E, Im JH, Kim SW, Yoon NS, Sung H, Kim MN, et al. Evaluation of combination of BACTEC mycobacteria growth indicator tube 960 system and Ogawa media for mycobacterial culture. Korean J Lab Med. 2008. 28:299–306.
Article17. Kim BJ, Lee IH, Lee DH, Bai GH, Kong SJ, Lee SH, et al. The current status of multidrug-resistant tuberculosis in Korea. Tuberc Respir Dis. 2006. 60:404–411.
Article18. Oh SH, Kim YJ, Park SK, Hwang SH, Kim HH, Lee EY, et al. Comparison of anti-mycobacterial drug susceptibility test results by institutes and methods. Korean J Clin Microbiol. 2008. 11:43–48.
Article19. Han MD, Im JS, Yim J, Oh DK. A study on the drug susceptibility test of multi-drug resistant tuberculosis patients. Korean J Epidemiol. 2008. 30:301–308.
Article20. Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003. 167:603–662.21. Joh JS, Lee CH, Lee JE, Park YK, Bai GH, Kim EC, et al. The interval between initiation of anti-tuberculosis treatment in patients with culture-positive pulmonary tuberculosis and receipt of drug-susceptibility test results. J Korean Med Sci. 2007. 22:26–29.
Article22. Abe C, Kobayashi I, Mitarai S, Wada M, Kawabe Y, Takashima T, et al. Biological and molecular characteristics of Mycobacterium tuberculosis clinical isolates with low-level resistance to isoniazid in Japan. J Clin Microbiol. 2008. 46:2263–2268.23. Van Deun A, Barrera L, Bastian I, Fattorini L, Hoffmann H, Kam KM, et al. Mycobacterium tuberculosis strains with highly discordant rifampin susceptibility test results. J Clin Microbiol. 2009. 47:3501–3506.24. Van Deun A, Martin A, Palomino JC. Diagnosis of drug-resistant tuberculosis: reliability and rapidity of detection. Int J Tuberc Lung Dis. 2010. 14:131–140.25. Bai GH, Kim SJ, Chang CL. Proficiency analysis of drug susceptibility testing by national-level tuberculosis reference laboratories from 1995 to 2003. J Clin Microbiol. 2007. 45:3626–3630.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of Mycobacterium tuberculosis using BACTEC Mycobacteria Growth Indicator Tube(MGIT) 960 system: Comparison with BACTEC 460 TB system and Ogawa Media
- Evaluation of the BACTEC MGIT 960 system for the recovery of mycobacteria
- Evaluation of MGIT 960 System for Recovery of Mycobacteria from Body Fluids
- Evaluation of Mycobacteria Growth Indicator Tube for Drug Susceptibility Testing of Mycobacterium tuberculosis Using MGIT 960 System
- Comparison of Mycobacterial Culture by Mycobacterium Growth Indicator Tube and Ogawa Media