J Vet Sci.
2011 Dec;12(4):405-407. 10.4142/jvs.2011.12.4.405.
Post-mortem re-cloning of a transgenic red fluorescent protein dog
- Affiliations
-
- 1Department of Theriogenology and Biotechnology, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. bclee@snu.ac.kr
- KMID: 1365026
- DOI: http://doi.org/10.4142/jvs.2011.12.4.405
Abstract
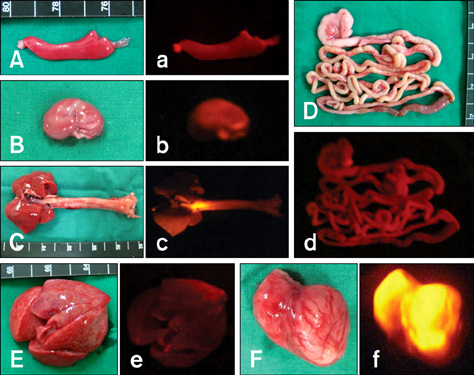
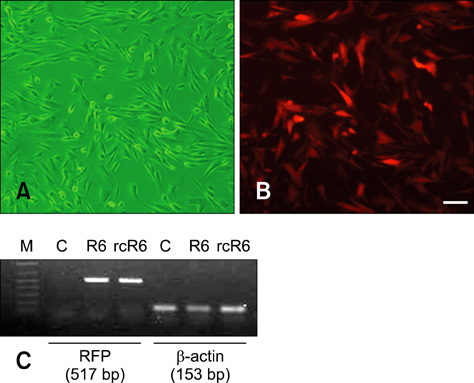
- Recently, the world's first transgenic dogs were produced by somatic cell nuclear transfer. However, cellular senescence is a major limiting factor for producing more advanced transgenic dogs. To overcome this obstacle, we rejuvenated transgenic cells using a re-cloning technique. Fibroblasts from post-mortem red fluorescent protein (RFP) dog were reconstructed with in vivo matured oocytes and transferred into 10 surrogate dogs. One puppy was produced and confirmed as a re-cloned dog. Although the puppy was lost during birth, we successfully established a rejuvenated fibroblast cell line from this animal. The cell line was found to stably express RFP and is ready for additional genetic modification.
MeSH Terms
-
Animals
Animals, Genetically Modified
Cloning, Organism/methods/*veterinary
Dogs/*genetics
Female
Gastrointestinal Tract/metabolism
Gene Expression Regulation
Kidney/metabolism
Liver/metabolism
Luminescent Proteins/*genetics/metabolism
Lung/metabolism
Male
Myocardium/metabolism
Nuclear Transfer Techniques/veterinary
Spleen/metabolism
Trachea/metabolism
Figure
Reference
-
1. Cho SJ, Bang JI, Yu XF, Lee YS, Kim JH, Jeon JT, Yee ST, Kong IK. Generation of a recloned transgenic cat expressing red fluorescence protein. Theriogenology. 2010. 73:848–855.
Article2. Cho SK, Kim JH, Park JY, Choi YJ, Bang JI, Hwang KC, Cho EJ, Sohn SH, Uhm SJ, Koo DB, Lee KK, Kim T, Kim JH. Serial cloning of pigs by somatic cell nuclear transfer: restoration of phenotypic normality during serial cloning. Dev Dyn. 2007. 236:3369–3382.
Article3. Cibelli JB, Stice SL, Golueke PJ, Kane JJ, Jerry J, Blackwell C, Ponce de León FA, Robl JM. Cloned transgenic calves produced from nonquiescent fetal fibroblasts. Science. 1998. 280:1256–1258.
Article4. Fujimura T, Murakami H, Kurome M, Takahagi Y, Shigehisa T, Nagashima H. Effects of recloning on the efficiency of production of α1,3-galactosyltransferase knockout pigs. J Reprod Dev. 2008. 54:58–62.
Article5. Hill JR, Winger QA, Long CR, Looney CR, Thompson JA, Westhusin ME. Development rates of male bovine nuclear transfer embryos derived from adult and fetal cells. Biol Reprod. 2000. 62:1135–1140.
Article6. Hong SG, Kim MK, Jang G, Oh HJ, Park JE, Kang JT, Koo OJ, Kim T, Kwon MS, Koo BC, Ra JC, Kim DY, Ko C, Lee BC. Generation of red fluorescent protein transgenic dogs. Genesis. 2009. 47:314–322.
Article7. Kubota C, Tian XC, Yang X. Serial bull cloning by somatic cell nuclear transfer. Nat Biotechnol. 2004. 22:693–694.
Article8. Lee BC, Kim MK, Jang G, Oh HJ, Yuda F, Kim HJ, Hossein MS, Kim JJ, Kang SK, Schatten G, Hwang WS. Dogs cloned from adult somatic cells. Nature. 2005. 436:641.
Article9. Oh HJ, Hong SG, Park JE, Kang JT, Kim MJ, Kim MK, Kang SK, Kim DY, Jang G, Lee BC. Improved efficiency of canine nucleus transfer using roscovitine-treated canine fibroblasts. Theriogenology. 2009. 72:461–470.
Article10. Oh HJ, Kim MK, Jang G, Kim HJ, Hong SG, Park JE, Park K, Park C, Sohn SH, Kim DY, Shin NS, Lee BC. Cloning endangered gray wolves (Canis lupus) from somatic cells collected postmortem. Theriogenology. 2008. 70:638–647.
Article11. Parker HG, Kruglyak L, Ostrander EA. Molecular genetics: DNA analysis of a putative dog clone. Nature. 2006. 440:E1–E2.12. Van Thuan N, Kishigami S, Wakayama T. How to improve the success rate of mouse cloning technology. J Reprod Dev. 2010. 56:20–30.
Article13. Yin XJ, Lee HS, Yu XF, Kim LH, Shin HD, Cho SJ, Choi EG, Kong IK. Production of second-generation cloned cats by somatic cell nuclear transfer. Theriogenology. 2008. 69:1001–1006.
Article14. Zakhartchenko V, Mueller S, Alberio R, Schernthaner W, Stojkovic M, Wenigerkind H, Wanke R, Lassnig C, Mueller M, Wolf E, Brem G. Nuclear transfer in cattle with non-transfected and transfected fetal or cloned transgenic fetal and postnatal fibroblasts. Mol Reprod Dev. 2001. 60:362–369.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Improved development of somatic cell cloned bovine embryos by a mammary gland epithelia cells in vitro model
- Studies of cocktail therapy with multiple cytokines for neoplasia or infectious disease of the dog I. cDNA cloning of canine IL-3 and IL-6
- Health and temperaments of cloned working dogs
- Two cases of cat-pork syndrome in adults
- Reproductive ability of a cloned male detector dog and behavioral traits of its offspring