J Vet Sci.
2011 Dec;12(4):303-307. 10.4142/jvs.2011.12.4.303.
Biodynamic parameters of micellar diminazene in sheep erythrocytes and blood plasma
- Affiliations
-
- 1Saratov State Agrarian University, Saratov 410012, Russia. staroverov@ibppm.sgu.ru
- 2Institute of Biochemistry and Physiology of Plants and Microorganisms, Russian Academy of Sciences, Saratov 410049, Russia.
- KMID: 1365012
- DOI: http://doi.org/10.4142/jvs.2011.12.4.303
Abstract
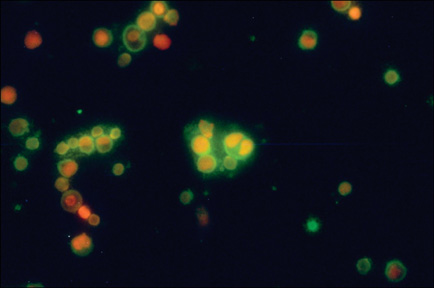
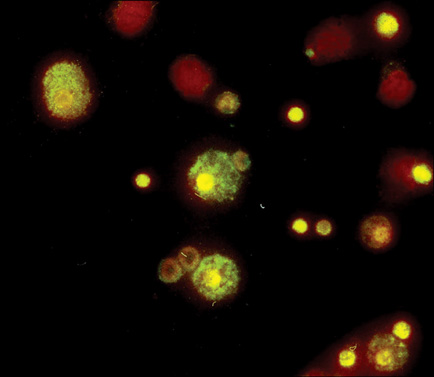
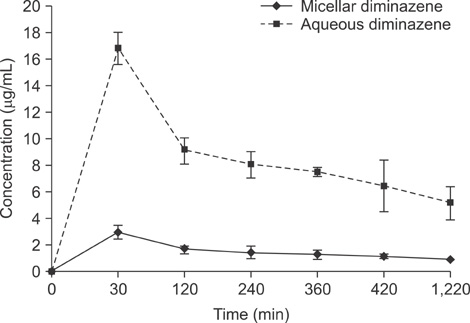
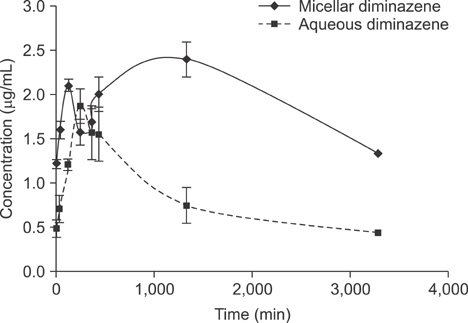
- In this work, we used a preparation of diminazene, which belongs to the group of aromatic diamidines. This compound acts on the causative agents of blood protozoan diseases produced by both flagellated protozoa (Trypanosoma) and members of the class Piroplasmida (Babesia, Theileria, and Cytauxzoon) in various domestic and wild animals, and it is widely used in veterinary medicine. We examined the behavior of water-disperse diminazene (immobilized in Tween 80 micelles) at the cellular and organismal levels. We assessed the interaction of an aqueous and a water-disperse preparation with cells of the reticuloendothelial system. We compared the kinetic parameters of aqueous and water-disperse diminazene in sheep erythrocytes and plasma. The therapeutic properties of these two preparations were also compared. We found that the surface-active substances improved intracellular penetration of the active substance through interaction with the cell membrane. In sheep blood erythrocytes, micellar diminazene accumulated more than its aqueous analog. This form was also more effective therapeutically than the aqueous analog. Our findings demonstrate that use of micellar diminazene allows the injection dose to be reduced by 30%.
Keyword
MeSH Terms
Figure
Reference
-
1. Aboofazeli R, Lawrence MJ. Investigations into the formation and characterization of phospholipid microemulsions. I. Pseudo-ternary phase diagrams of systems containing water-lecithin-alcohol-isopropyl myristate. Int J Pharm. 1993. 93:161–175.
Article2. Campbell M, Prankerd RJ, Davie AS, Charman WN. Degradation of berenil a (diminazene aceturate) in acidic aqueous solution. J Pharm Pharmacol. 2004. 56:1327–1332.3. Çomoğlu T, Gönül N. Microemulsions. J Fac Pharm Ankara. 1997. 26:95–108.4. Davenas E, Poitevin B, Benveniste J. Effect on mouse peritoneal macrophages of orally administered very high dilutions of silica. Eur J Pharmacol. 1987. 135:313–319.
Article5. Fubini B, Gasco MR, Gallarate M. Microcalorimetric study of microemulsions as potential drug delivery systems. II. Evaluation of enthalpy in the presence of drugs. Int J Pharm. 1989. 50:213–217.
Article6. Homer MJ, Aguilar-Delfin I, Telford SR III, Krause PJ, Persing DH. Babesiosis. Clin Microbiol Rev. 2000. 13:451–469.
Article7. Karvonen E, Kauppinen L, Partanen T, Pösö H. Irreversible inhibition of putrescine-stimulated S-adenosyl-L-methionine decarboxylase by Berenil and Pentamidine. Biochem J. 1985. 231:165–169.
Article8. Klatt P, Hajdu P. Pharmacokinetic investigations on diminazene and rolitetracycline in comparison to a combination of both. Vet Rec. 1976. 99:372–374.
Article9. Kwon GS. Polymeric micelles for delivery of poorly water-soluble compounds. Crit Rev Ther Drug Carrier Syst. 2003. 20:357–403.
Article10. Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004. 428:487–492.
Article11. Loginova NV, Polozov GI. An Introduction to Pharmaceutical Chemistry. 2003. Minsk: BGU Publishing House;70–72. [in Russian].12. Men'shikov VV. Clinical Laboratory Analytics. Vol. II. Special Analytical Technologies in Clinical Laboratory. 1999. Moscow: Labinform-RAMLD Publishing House;17. [in Russian].13. Munshi N, Rapoport N, Pitt WG. Ultrasonic activated drug delivery from pluronic P-105 micelles. Cancer Lett. 1997. 118:13–19.
Article14. Osborne DU, Ward AJ, O'neill KJ. Microemulsions as topical drug delivery vehicles. I. Characterization of a model system. Drug Dev Ind Pharm. 1988. 14:1203–1219.
Article15. Pattarino F, Carlotti ME, Gasco MR. Topical delivery systems for azelaic acid: effect of the suspended drug in microemulsion. Pharmazie. 1994. 49:72–73.16. Penzhorn BL, Lewis BD, de Waal DT, López Rebollar LM. Sterilisation of Babesia canis infections by imidocarb alone or in combination with diminazene. J S Afr Vet Assoc. 1995. 66:157–159.17. Seddon JM, Templer RH. Hoff AJ, Lipowsky R, Sackmann E, editors. Polymorphism of lipid-water systems. Handbook of Biological Physics. 1995. Vol. 1. Amsterdam: Elsevier;97–160. Chap. 3.
Article18. Staroverov SA, Pristensky DV, Yermilov DN, Gabalov KP, Zhemerichkin DA, Sidorkin VA, Shcherbakov AA, Shcyogolev SY, Dykman LA. The effectivity analysis of accumulation of liposomal, micellar, and water-soluble forms of diminazene in cells and in organs. Drug Deliv. 2006. 13:351–355.
Article19. Staroverov SA, Sidorkin VA, Yermilov DN, Vasilenko OA, Ulizko MA. Pharmacodynamics of a new diminazene drug form. Veterinary. 2005. 5:49–51. [in Russian].20. Storz H. Friemel H, editor. Immunofluorescene. Immunologische Methods. 1987. Moscow: Meditsina Publishing House;128–131. [in Russian].21. Woodburn K, Kessel D. The alteration of plasma lipoproteins by cremophor EL. J Photochem Photobiol B. 1994. 22:197–201.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunohistochemical study on the formation of germinal centers in the spleen of mouse injected with sheep red blood cells
- Cerebellar encephalopathy from diminazene aceturate (beneril) toxicity in a dog
- Effect of Eveing Primrose on Plasma Cholesterol Levels and Immune Response to Sheep Erythrocytes
- Effect of Cu Zn Levels on Superoxide Dismutase Activity in Erythrocytes from Patients with end Stage Renal Disease
- T-Lymphocyte Fluctuation in Patients with Primary Intracranial Tumors