Korean J Urol.
2008 May;49(5):432-438. 10.4111/kju.2008.49.5.432.
Effects of Mesenchymal Stem Cells on Stress Incontinence in a Rat Model
- Affiliations
-
- 1Department of Urology, Chonnam National University Medical School, Gwangju, Korea. urokwon@gmail.com
- 2Department of Cardiovascular Medicine, Chonnam National University Medical School, Gwangju, Korea.
- 3Department of Pathology, Chonnam National University Medical School, Gwangju, Korea.
- KMID: 1204524
- DOI: http://doi.org/10.4111/kju.2008.49.5.432
Abstract
-
PURPOSE: Several study trials have used stem cells to treat stress incontinence in an animal model. In this study, we compared injecting either periurethral mesenchymal stem cells(MSC) or normal saline(C) to increase the leak point pressure(LPP) and closing pressure(CP) in a rat model of stress urinary incontinence.
MATERIALS AND METHODS
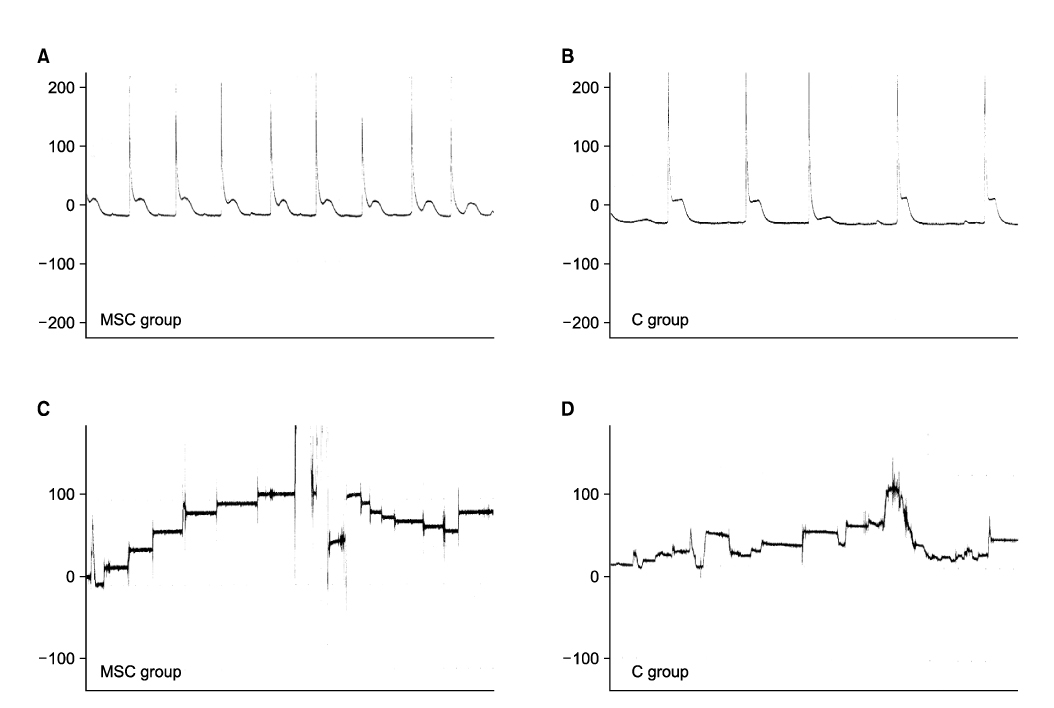
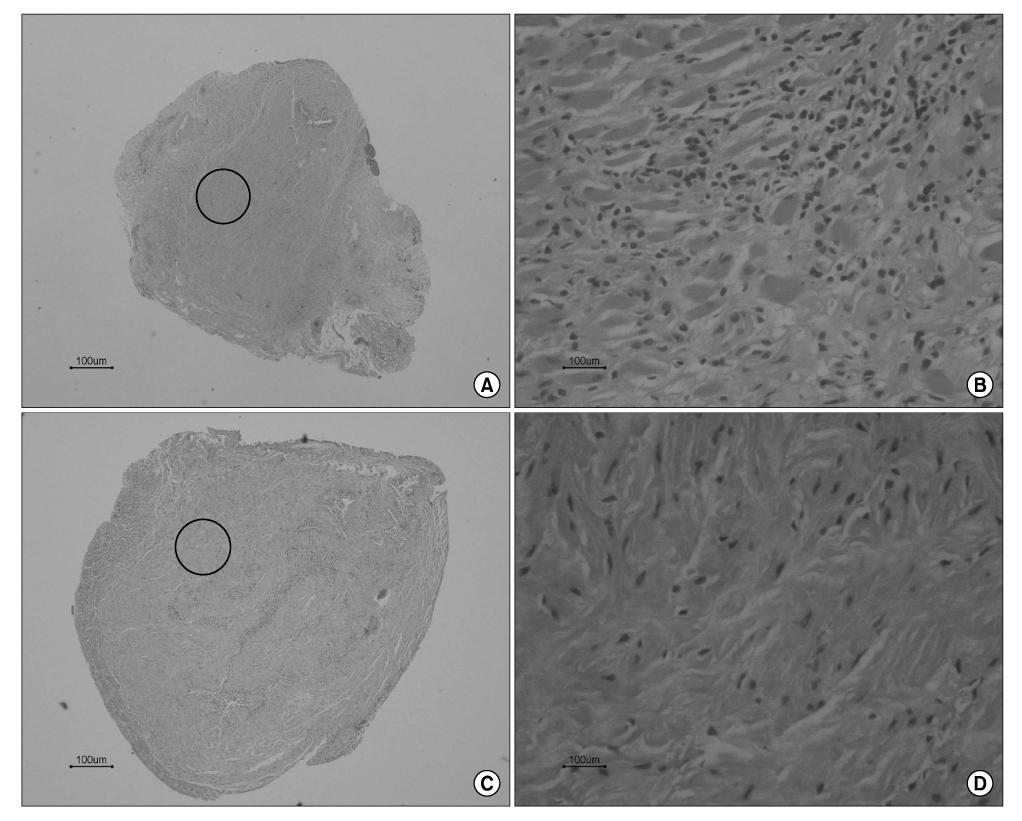
Sprague Dawley rats(250g each, 12 weeks old) were divided into the MSC group(n=5) and group C(n=5). They were anesthetized and the pudendal nerve was transected bilaterally via a ventral incision in order to denervate the external urethral sphincter. The MSCs were obtained from both femurs of Sprague Dawley rats(150g each, 6 weeks, n=10). After 1 week, the MSCs were stained by 4'-6-diamidino- 2-phenylindole(DAPI), which was injected into both sites of the proximal external urethra(n=1.5x10(6)). At 3 weeks after injection, cystometry was performed and this was followed by cord transection at the T9-10 level with the rat under anesthesia. Visually identified LPP and CP measurements were evaluated with using a vertical tilt/intravesical pressure clamp. The urethral tissues of the rats were harvested for histology.
RESULTS
Both the LPP and CP measurements were significantly higher in the MSC group when compared with that of the C group(p<0.05). The mean LPP of the MSC group and group C was 42.3+/-2.1cmH2O and 25.8+/-1.7cmH2O, respectively. The mean CP of the MSC group and group C was 31.7+/-2.5cmH2O and 21.3+/-1.1cmH2O, respectively. The existence of DAPI-stained MSCs in the injected periurethral tissue was verified by histology after the completion of the study.
CONCLUSIONS
Injection of MSCs into the periurethal tissue after transection of the bilateral pudendal nerve in rats led to an increase in the LPP and CP. This finding suggests that MSCs can be used as one of the potentially effective cell therapies for stress urinary incontinence.
Figure
Reference
-
1. Mah SY, Lee KS, Choo MS, Seo JT, Lee JZ, Park WH, et al. Duloxetine versus placebo for the treatment of Korean women with stress predominant urinary incontinence. Korean J Urol. 2006. 47:527–535.2. Cannon TW, Chancellor MB. Pharmacotherapy for stress urinary incontinence. Rev Urol. 2003. 5:135–141.3. Henly DR, Barrett DM, Weiland TL, O'Connor MK, Malizia AA, Wein AJ. Particulate silicon for use in periurethral injections: a study of local tissue effects and search for migration. J Urol. 1992. 147:376A.4. Kuuva N, Nilsson CG. A nationwide analysis of complications associated with the tension-free vaginal tape (TVT) procedure. Acta Obstet Gynecol Scand. 2002. 81:72–77.5. Levin I, Groutz A, Gold R, Pauzner D, Lessing JB, Gordon D. Surgical complications and medium-term outcome results of tension-free vatinal tape: a prospective study of 313 consecutive patients. Neurourol Urodyn. 2004. 23:7–9.6. Chancellor MB, Yokoyama T, Tirney S, Mattes CE, Ozawa H, Yoshimura N, et al. Preliminary results of myoblast injection into the urethra and bladder wall: a possible method for the treatment of stress urinary incontinence and impaired detrusor contractility. Neurourol Urodyn. 2000. 19:279–287.7. Chermansky CJ, Tarin T, Kwon DD, Jankowski RJ, Cannon TW, de Groat WC, et al. Intraurethral muscle-derived cell injections increase leak point pressure in a rat model of intrinsic sphincter deficiency. Urology. 2004. 63:780–785.8. Lee JW, Kim YH, Kim SH, Han SH, Hahn SB. Chondrogenic differentiation of mesenchymal stem cells and its clinical applications. Yonsei Med J. 2004. 45:Suppl. 41–47.9. Kane DD, Shott S, Hughes WF, Kerns JM. Motor pudendal nerve characterization in the female rat. Anat Rec. 2002. 266:21–29.10. Juma S, Little NA, Raz S. Vaginal wall sling: four years later. Urology. 1992. 39:424–428.11. Oh SJ, Park WH, Park CH, Paick JS, Seo JT, Lee YS, et al. Prevalence of urinary incontinence and incontinence-related quality of life in Korean women: a population-based study. J Korean Continence Soc. 2003. 7:73–80.12. Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994. 125:1275–1287.13. Yokoyama T, Huard J, Chancellor MB. Myoblast therapy for stress urinary incontinence and bladder dysfunction. World J Urol. 2000. 18:56–61.14. Manzo J, Vazquez MI, Cruz MR, Hernandez ME, Carrillo P, Pacheco P. Fertility ratio in male rats: effects after denervation of two pelvic floor muscles. Physiol Behav. 2000. 68:611–618.15. Cannon TW, Lee JY, Somogyi G, Prunchnic R, Smith CP, Huard J, et al. Improved sphincter contractility after allogenic muscle-derived progenitor cell injection into the denervated rat urethra. Urology. 2003. 62:958–963.16. Yokoyama T, Yoshimura N, Dhir R, Qu Z, Fraser MO, Kumon H, et al. Persistence and survival of autologous muscle derived cells versus bovine collagen as potential treatment of stress urinary incontinence. J Urol. 2001. 165:271–276.17. Kwon D, Kim Y, Pruchnic R, Jankowski R, Usiene I, de Miguel F, et al. Periurethral cellular injection: comparison of muscle-derived progenitor cells and fibroblasts with regard to efficacy and tissue contractility in an animal model of stress urinary incontinence. Urology. 2006. 68:449–454.18. Strasser H, Marksteiner R, Margreiter E, Pinggera GM, Mitterberger M, Fritsch H, et al. Stem cell therapy for urinary incontinence. Urologe A. 2004. 43:1237–1241.19. Seivert KD, Amend B, Renninger M, Selent C, Feil G, Hennenlotter J, et al. Value of stem cell therapy for the treatment of stress incontinence. Current status and perspectives. Urologe A. 2007. 46:264–267.20. Becker C, Jakse G. Stem cells for regeneration of urological structures. Eur Urol. 2007. 51:1217–1228.21. Vilquin JT. Myoblast transplantation: clinical trials and perspectives. Mini-review. Acta Myol. 2005. 24:119–127.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- L-Theanine-Treated Adipose-Derived Mesenchymal Stem Cells Alleviate the Cytotoxicity Induced by N-Nitrosodiethylamine in Liver
- Combination Cell Therapy with Mesenchymal Stem Cells and Neural Stem Cells for Brain Stroke in Rats
- Effects of Mesenchymal Stem Cells Treatment on Radiation-Induced Proctitis in Rats
- Effects of Injection Therapy using Muscle Derived Stem Cell/Chitosan/Hydroapatite Composite Gel in a Rat Model of Urinary Incontinence
- Role of Mesenchymal Stem Cells in Patients with Nontraumatic Osteonecrosis of the Femoral Head