Yonsei Med J.
2010 Mar;51(2):239-243. 10.3349/ymj.2010.51.2.239.
Antibody Status in Children with Steroid-Sensitive Nephrotic Syndrome
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea. leekyungyil@catholic.ac.kr
- KMID: 1126024
- DOI: http://doi.org/10.3349/ymj.2010.51.2.239
Abstract
- PURPOSE
The pathophysiology of hypogammaglobulinemia in nephrotic syndrome (NS) remains unknown. We evaluated the differences in the distribution of anti-bacterial antibodies and anti-viral antibodies, and those of immune antibodies and natural antibodies in steroid-sensitive NS.
MATERIALS AND METHODS
We examined the antibody status of 18 children who had routine vaccinations. The levels of immnunoglobulin G (IgG), the IgG subclasses, and the antibodies induced by vaccinations such as diphtheria-pertussis-tetanus and measles-mumpsrubella were analyzed in children with steroid-sensitive NS.
RESULTS
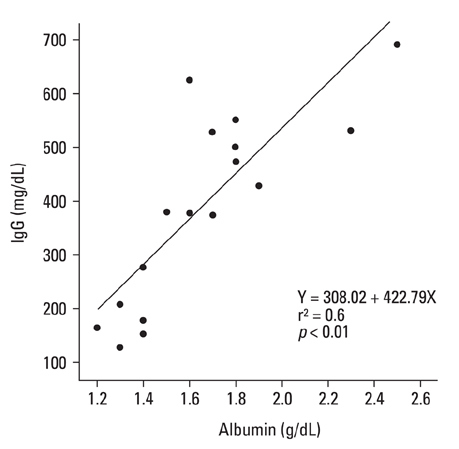
There was a positive correlation between the albumin and IgG values (r = 0.6, p < 0.01), and the four IgG subclasses were all evenly depressed in the nephrotic children during the acute stage of the disease. The antibodies induced by bacterial antigens were depressed and the seropositivity of anti-viral antibodies tended to be lower than those of age-matched control children during the acute stage. The depressed immune antibody status recovered rapidly in the remission stage of NS, despite corticosteroid treatment.
CONCLUSIONS
IgG levels correlated positively with albumin levels, and all antibodies, including immune and natural antibodies, were depressed in the acute stage of NS. Our results suggest that hypogammaglobulinaemia in NS may be associated with intravascular homeostasis of oncotic pressure.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
A Common Immunopathogenesis Mechanism for Infectious Diseases: The Protein-Homeostasis-System Hypothesis
Kyung-Yil Lee
Infect Chemother. 2015;47(1):12-26. doi: 10.3947/ic.2015.47.1.12.
Reference
-
1. Giangiacomo J, Cleary TG, Cole BR, Hoffsten P, Robson AM. Serum immunoglobulins in the nephrotic syndrome. A possible cause of minimal-change nephrotic syndrome. N Engl J Med. 1975. 293:8–12.2. Kemper MJ, Altrogge H, Ganschow R, Müller-Wiefel DE. Serum levels of immunoglobulins and IgG subclasses in steroid sensitive nephrotic syndrome. Pediatr Nephrol. 2002. 17:413–417.
Article3. Krensky AM, Ingelfinger JR, Grupe WE. Peritonitis in childhood nephrotic syndrome: 1970-1980. Am J Dis Child. 1982. 136:732–736.4. Brocklebank T, Cooper EH, Richmond K. Sodium dodecyl sulphate polyacrylamide gel electrophoresis patterns of proteinuria in various renal diseases of childhood. Pediatr Nephrol. 1991. 5:371–375.
Article5. Ramjee G, Coovadia HM, Adhikari M. Sodium dodecyl sulphate polyacrylamide gel electrophoresis of urinary proteins in steroidresponsive and steroid-resistant nephrotic syndrome in children. Pediatr Nephrol. 1994. 8:653–656.
Article6. Harris HW Jr, Umetsu D, Geha R, Harmon WE. Altered immunoglobulin status in congenital nephrotic syndrome. Clin Nephrol. 1986. 25:308–313.7. Huttunen NP, Savilahti E, Rapola J. Selectivity of proteinuria in congenital nephrotic syndrome of the Finnish type. Kidney Int. 1975. 8:255–261.
Article8. Beaman M, Oldfield S, MacLennan IC, Michael J, Adu D. Hypogammaglobulinaemia in nephrotic rats is attributable to hypercatabolism of IgG. Clin Exp Immunol. 1988. 74:425–430.9. Heslan JM, Lautie L, Intrator C, Blanc C, Lagrue G, Sobel AT. Impaired IgG synthesis in patients with the nephrotic syndrome. Clin Nephrol. 1982. 18:144–147.10. Warshaw BL, Check IJ, Hymes LC, DiRusso SC. Decreased serum transferrin concentration in children with the nephrotic syndrome: effect on lymphocyte proliferation and correlation with serum immunoglobulin levels. Clin Immunol Immunopathol. 1984. 33:210–219.11. Kwak GY, Lee KY, Kim DU, Koh DK, Lee JS. Correlation between serum albumin and IgG level in minimal change nephrotic syndrome. J Korean Soc Pediatr Nephrol. 2007. 11:16–23.12. Siber GR, Schur PH, Aisenberg AC, Weitzman SA, Schiffman G. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N Engl J Med. 1980. 303:178–182.
Article13. Linde GA. Subclass distribution of rubella virus-specific immunoglobulin G. J Clin Microbiol. 1985. 21:117–121.
Article14. Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med. 2001. 345:747–755.
Article15. Lee KY, Lee JS. Immunoglobulin G has a role for systemic protein modulation in vivo: a new concept of protein homeostasis. Med Hypotheses. 2006. 67:848–855.
Article16. Sibéril S, Elluru S, Negi VS, Ephrem A, Misra N, Delignat S, et al. Intravenous immunoglobulin in autoimmune and inflammatory diseases: more than mere transfer of antibodies. Transfus Apher Sci. 2007. 37:103–107.17. al-Bander HA, Martin VI, Kaysen GA. Plasma IgG pool is not defended from urinary loss in nephrotic syndrome. Am J Physiol. 1992. 262:F333–F337.
Article18. Kaysen GA. Plasma composition in the nephrotic syndrome. Am J Nephrol. 1993. 13:347–359.
Article19. Lee KY, Han JW, Lee JS. Kawasaki diasease may be a hyperimmune reaction of genetically susceptible children to variants of normal environmental flora. Med Hypotheses. 2007. 69:642–651.
Article20. Orbach H, Tishler M, Shoenfeld Y. Intravenous immunoglobulin and the kidney--a two-edged sword. Semin Arthritis Rheum. 2004. 34:593–601.
Article21. Warshaw BL, Check IJ. IgG subclasses in children with nephrotic syndrome. Am J Clin Pathol. 1989. 92:68–72.
Article22. Kimata H, Fujimoto M, Furusho K. Involvement of interleukin (IL)-13, but not IL-4, in spontaneous IgE and IgG4 production in nephrotic syndrome. Eur J Immunol. 1995. 25:1497–1501.23. Rostoker G, Pech MA, Del Prato S, Petit-Phar M, Ben Maadi A, Dubert JM, et al. Serum IgG subclasses and IgM imbalances in adult IgA mesangial glomerulonephritis and idiopathic Henoch-Schoenlein purpura. Clin Exp Immunol. 1989. 75:30–34.24. Imai H, Hamai K, Komatsuda A, Ohtani H, Miura AB. IgG subclasses in patients with membranoproliferative glomerulonephritis, membranous nephropathy, and lupus nephritis. Kidney Int. 1997. 51:270–276.
Article25. Wilkes JC, Nelson JD, Worthen HG, Morris M, Hogg RJ. Response to pneumococcal vaccination in children with nephrotic syndrome. Am J Kidney Dis. 1982. 2:43–46.26. Tejani A, Fikrig S, Schiffman G, Gurumurthy K. Persistence of protective pneumococcal antibody following vaccination in patients with the nephrotic syndrome. Am J Nephrol. 1984. 4:32–37.
Article27. Cho MH, Hong EH, Lee TH, Ko CW. Pathophysiology of minimal change nephrotic syndrome and focal segmental glomerulosclerosis. Nephrology (Carlton). 2007. 12:Suppl 3. S11–S14.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of C1q Nephropathy in Steroid-Dependent Nephrotic Syndrome
- Levamisole-Induced Reversible Agranulocytosis in Children with Steroid Dependent Nephrotic Syndrome
- Can We Predict How Often Nephrotic Syndrome will Relapse into the Patients?
- A Case of Levamisole Treatment for Kimura's Disease-Associated Nephrotic Syndrome
- Clinical Review of Idiopathic Nephrotic Syndrome in Children