Yonsei Med J.
2010 Mar;51(2):225-230. 10.3349/ymj.2010.51.2.225.
Clinical Heterogeneity in Korean Patients with Nemaline Myopathy
- Affiliations
-
- 1Department of Neurology, Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea. ycchoi@yuhs.ac
- 2Department of Pathology, Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Neurology, The Catholic University of Korea College of Medicine, Seoul, Korea.
- KMID: 1126022
- DOI: http://doi.org/10.3349/ymj.2010.51.2.225
Abstract
- PURPOSE
Nemaline myopathy (NM) is a clinical heterogeneous congenital myopathy characterized by the presence of subsarcolemmal or cytoplasmic rod-like structures that call nemaline bodies in the muscle fibers. The purpose of this study was to investigate the clinical diversity and pathological features of Korean patients with NM.
MATERIALS AND METHODS
Eight patients underwent analyses of clinical manifestations by a structured protocol. Diagnoses were established by a muscle biopsy.
RESULTS
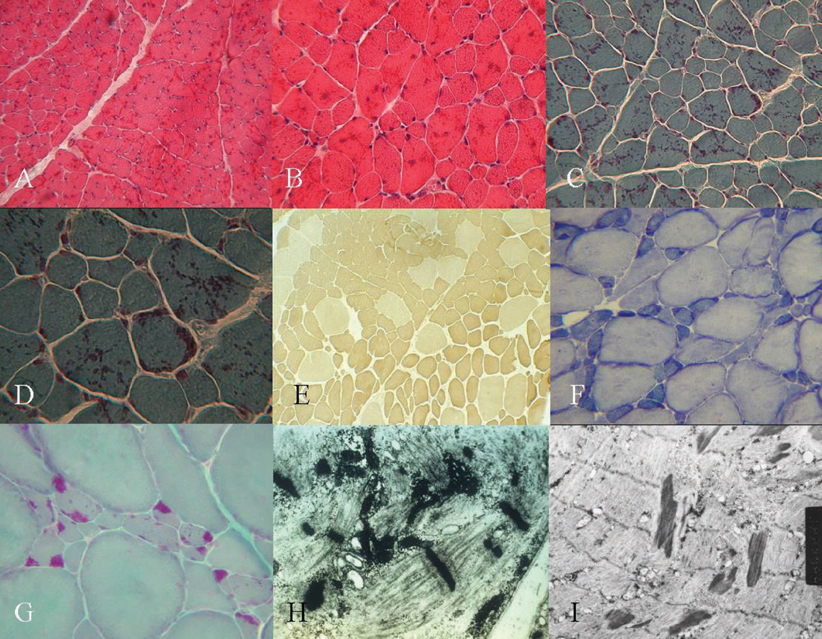
Two patients had the typical congenital type, which exhibited neonatal hypotonia and delayed motor milestone, and five patients had the childhood onset type, which exhibited mild gait disturbance as a first symptom. One patient had the adult onset type, which showed acute respiratory failure. Limb weakness was proximal-dominant occurred in six patients. Hyporeflexia was observed in most patients. Elongated faces and high arched palates and feet were also observed. On light microscopy, the nemaline bodies were observed in type 1 and 2 fibers. All patients showed type 1 predominance and atrophy. In the two cases in which ultrastructural studies were performed, typical nemaline rods and disorganized myofibrillar apparatus were detected.
CONCLUSION
In conclusion, the eight Korean patients in this study with NM shared common clinical expressions such as proximal limb weakness, reduced deep tendon reflex, and dysmorphic features. This study, however, showed that clinical heterogeneity ranged from typical congenital, mildly affected childhood to the adult onset form with acute respiratory failure. The pathological findings in this study were in accordance with those of other previous reports.
MeSH Terms
Figure
Cited by 1 articles
-
Nemaline Myopathy Presenting as Adult-onset Distal Myopathy
Kee Hong Park, Sung-Yeon Sohn, Je-Young Shin, Jun-Soon Kim, Sung-Hye Park, Ji-Sun Kim, Jung-Joon Sung
Korean J Clin Neurophysiol. 2016;18(1):31-33. doi: 10.14253/kjcn.2016.18.1.31.
Reference
-
1. Agrawal PB, Strickland CD, Midgett C, Morales A, Newburger DE, Poulos MA, et al. Heterogeneity of nemaline myopathy cases with skeletal muscle alpha-actin gene mutations. Ann Neurol. 2004. 56:86–96.
Article2. Wallgren-Pettersson C, Laing NG. Report of the 83th ENMC International Workshop: 4th Workshop on Nemaline Myopathy, 22-24 September 2000, Naarden, The Netherlands. Neuromuscul Disord. 2001. 11:589–595.3. Ryan MM, Schnell C, Strickland CD, Shield LK, Morgan G, Iannaccone ST, et al. Nemaline myopathy: a clinical study of 143 cases. Ann Neurol. 2001. 50:312–320.
Article4. Wallgren-Pettersson C, Laing NG. Report of the 70th ENMC International Workshop: nemaline myopathy, 11-13 June 1999, Naarden, The Netherlands. Neuromuscul Disord. 2000. 10:299–306.
Article5. Jungbluth H, Sewry CA, Muntoni F. What's new in neuromuscular disorders? The congenital myopathies. Eur J Paediatr Neurol. 2003. 7:23–30.
Article6. Katirji B, Kaminski HJ, Preston DC, Ruff RL, Shapiro BE. Bodensteiner JB, Riggs JE, Schochet SS, editors. Neuromuscular disorders in clinical practice. Congenital myopathies. 2002. 1st ed. Woburn: Butterworth-Heinemann;1114–1127.7. Conen PE, Murphy EG, Donohue WL. Light and electron microscopic studies of "myogranules" in a child with hypotonia and muscle weakness. Can Med Assoc J. 1963. 89:983–986.8. Shy GM, Engel WK, Somers JE, Wanko T. Nemaline myopathy. A new congenital myopathy. Brain. 1963. 86:793–810.9. Shimomura C, Nonaka I. Nemaline myopathy: comparative muscle histochemistry in the severe neonatal, moderate congenital, and adult-onset forms. Pediatr Neurol. 1989. 5:25–31.
Article10. Rifai Z, Kazee AM, Kamp C, Griggs RC. Intranuclear rods in severe congenital nemaline myopathy. Neurology. 1993. 43:2372–2377.
Article11. Sanoudou D, Beggs AH. Clinical and genetic heterogeneity in nemaline myopathy--a disease of skeletal muscle thin filaments. Trends Mol Med. 2001. 7:362–368.
Article12. Gyure KA, Prayson RA, Estes ML. Adult-onset nemaline myopathy: a case report and review of the literature. Arch Pathol Lab Med. 1997. 121:1210–1213.13. Nonaka I, Ishiura S, Arahata K, Ishibashi-Ueda H, Maruyama T, Ii K. Progression in nemaline myopathy. Acta Neuropathol. 1989. 78:484–491.
Article14. Gurgel-Giannetti J, Reed UC, Marie SK, Zanoteli E, Fireman MA, Oliveira AS, et al. Rod distribution and muscle fiber type modification in the progression of nemaline myopathy. J Child Neurol. 2003. 18:235–240.15. Volpe P, Damiani E, Margreth A, Pellegrini G, Scarlato G. Fast to slow change of myosin of nemaline myopathy: electrophoretic and immunologic evidence. Neurology. 1982. 32:37–41.16. Ryan MM, Ilkovski B, Strickland CD, Schnell C, Sanoudou D, Midgett C, et al. Clinical course correlates poorly with muscle pathology in nemaline myopathy. Neurology. 2003. 60:665–673.
Article17. Kaimaktchiev V, Goebel H, Laing N, Narus M, Weeks D, Nixon R. Intranuclear nemaline rod myopathy. Muscle Nerve. 2006. 34:369–372.18. Barohn RJ, Jackson CE, Kagan-Hallet KS. Neonatal nemaline myopathy with abundant intranuclear rods. Neuromuscul Disord. 1994. 4:513–520.
Article19. Goebel HH, Piirsoo A, Warlo I, Schofer O, Kehr S, Gaude M. Infantile intranuclear rod myopathy. J Child Neurol. 1997. 12:22–30.
Article20. Laing NG, Wilton SD, Akkari PA, Dorosz S, Boundy K, Kneebone C, et al. A mutation in the alphatropomyosin gene TPM3 associated with autosomal dominant nemaline myopathy. Nat Genet. 1995. 9:75–79.
Article21. Pelin K, Hilpelä P, Donner K, Sewry C, Akkari PA, Wilton SD, et al. Mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Proc Natl Acad Sci U S A. 1999. 96:2305–2310.
Article22. Nowak KJ, Wattanasirichaigoon D, Goebel HH, Wilce M, Pelin K, Donner K, et al. Mutations in the skeletal muscle alpha-actin gene in patients with actin myopathy and nemaline myopathy. Nat Genet. 1999. 23:208–212.
Article23. Donner K, Ollikainen M, Ridanpää M, Christen HJ, Goebel HH, de Visser M, et al. Mutations in the beta-tropomyosin (TPM2) gene--a rare cause of nemaline myopathy. Neuromuscul Disord. 2002. 12:151–158.
Article24. Johnston JJ, Kelley RI, Crawford TO, Morton DH, Agarwala R, Koch T, et al. A novel nemaline myopathy in the Amish caused by a mutation in troponin T1. Am J Hum Genet. 2000. 67:814–821.
Article25. Goebel HH. Congenital myopathies at their molecular dawning. Muscle Nerve. 2003. 27:527–548.
Article26. Chahin N, Selcen D, Engel AG. Sporadic late onset nemaline myopathy. Neurology. 2005. 65:1158–1164.
Article27. de Sanctis JT, Cumbo-Nacheli G, Dobbie D, Baumgartner D. HIV-associated nemaline rod myopathy: role of intravenous immunoglobulin therapy in two persons with HIV/AIDS. AIDS Read. 2008. 18:90–94.28. Colmegna I, Koehler JW, Garry RF, Espinoza LR. Musculoskeletal and autoimmune manifestations of HIV, syphilis and tuberculosis. Curr Opin Rheumatol. 2006. 18:88–95.
Article29. Shin JW, Park RJ, Aum SS, Choi TY. Analysis of anti-HIV positive rates and false-positive cases in a tertiary care hospital - single institute study. Korean J Blood Transfus. 2009. 20:40–45.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of the Dysphagia Therapy Child with Nemaline Myopathy Who Have Dysphagia: A Single Subject Research Design
- Electromyographic & clinical features of nemaline myopathy
- A Case of Nemaline Myopathy
- Anesthetic Experience of the Patient with Nemaline Myopathy: A case report
- A Korean Case of Neonatal Nemaline Myopathy Carrying KLHL40 Mutations Diagnosed Using Next Generation Sequencing