Korean J Lab Med.
2007 Feb;27(1):34-39. 10.3343/kjlm.2007.27.1.34.
Assessment of the Accuracy and Precision of Cystatin C-based GFR Estimates and Cr-based GFR Estimates in Comparison with Cr51-EDTA GFR
- Affiliations
-
- 1Department of Laboratory Medicine, Asan Medical Center and University of Ulsan College of Medicine, Seoul, Korea. wkmin@amc.seoul.kr
- 2Department of Nuclear Medicine, Asan Medical Center and University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 1121657
- DOI: http://doi.org/10.3343/kjlm.2007.27.1.34
Abstract
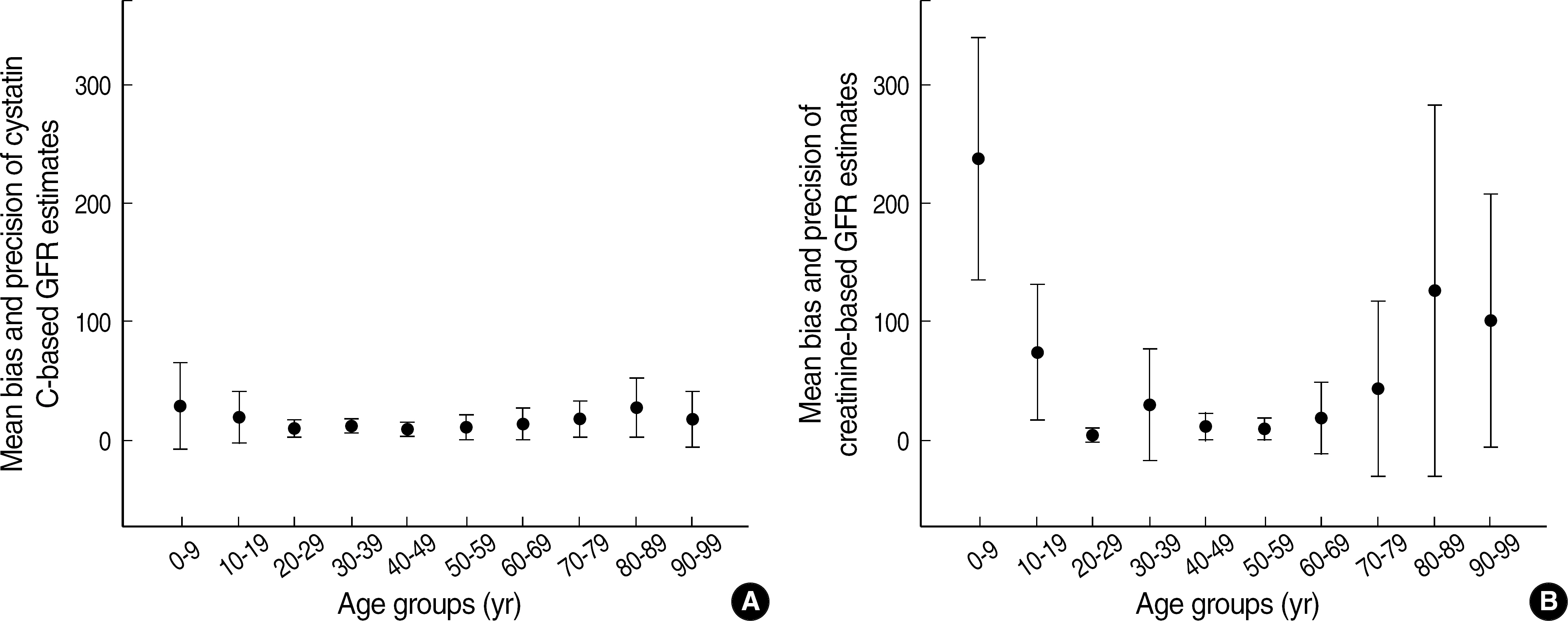
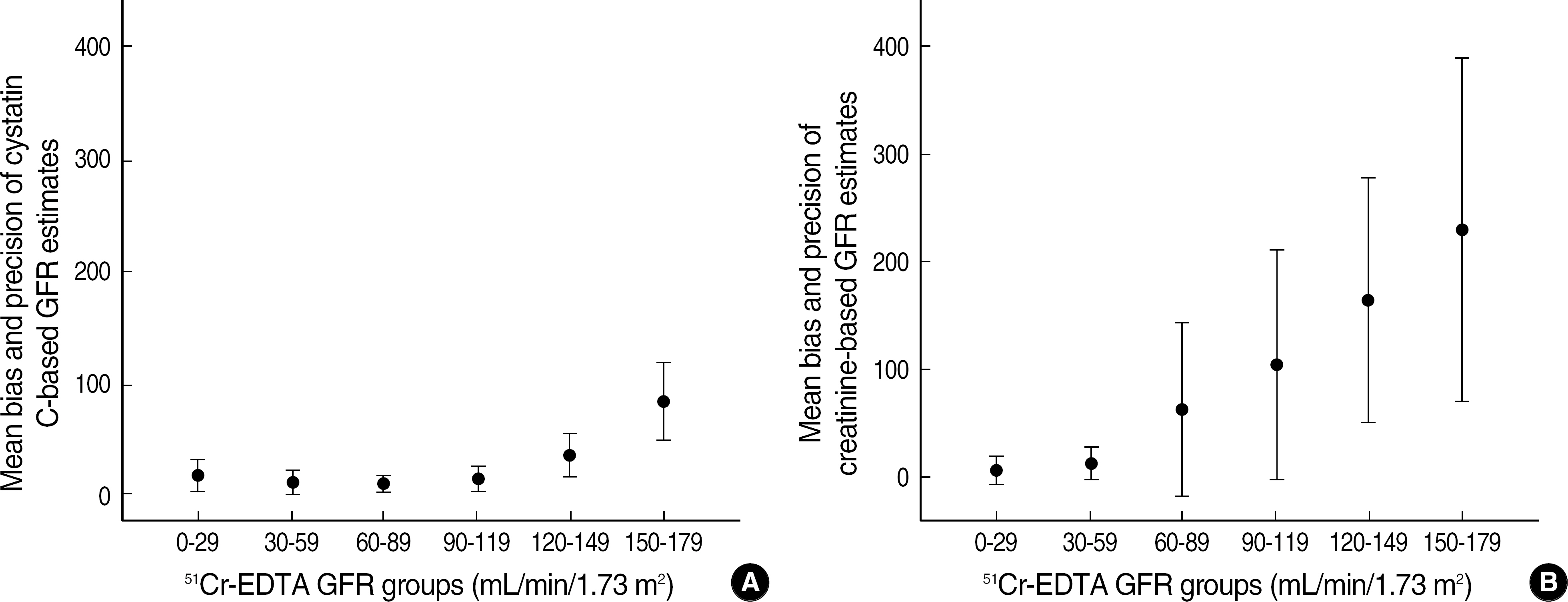
- BACKGROUND: Cystatin C (cysC) is said to be an ideal marker for glomerular filtration rate (GFR), independent of external factors such as age, nutrition and inflammation. The authors compared the accuracy and precision of cysC-based and creatinine (Cr)-based GFR estimates using Cr51-EDTA GFR method as a reference. METHODS: Serum concentrations of cysC and Cr were measured in adults over 17 yr (n=170) and children below 17 yr (n=79) who had had GFR estimated by Cr51-EDTA method. CysC-based GFR was estimated by the formula of Thierry [CysC-based GFR estimates (mL/min/1.73 m2)=78 x (1/cysC, in mg/L)+4] and Cr-based GFR by the formula of modified Modification of Diet in Renal Disease [MDRD II, Cr-based GFR estimates (mL/min/1.73 m2)=186 x (Scr)(-1.154) x (Age)(-0.203) x 0.742 (for a female patient) x 1.212 (for a black patient). RESULTS: In comparison with Cr51-EDTA GFR, in children below 17 yr, the bias +/- standard deviation (SD) of cysC-based and Cr-based GFR estimates were 7.5 +/- 6.1 and 106.5 +/- 98.2, respectively, in the range of below 90 of Cr51-EDTA GFR (mL/min/1.73 m2), and 33.7 +/- 33.0 and 174.4 +/- 18.8 in the range of over 90. In adults over 17 yr, the respective figures were 13.1 +/- 11.0 and 17.4 +/- 29.8 in below 90, and 21.2 +/- 20.1 and 83.6 +/- 108.8 in over 90 of Cr51-EDTA GFR. CONCLUSIONS: CysC-based GFR estimates show acceptable ranges of biases over the whole age and GFR ranges. CysC-based GFR estimates is considered to be the marker for GFR, which could be used without limitation of age and GFR ranges.
MeSH Terms
Figure
Reference
-
References
1. Lamb E, Newman DJ, Price CP. Kidney function tests. Burtis CA, Ashwood ER, Bruns ED, editors. eds.Tietz textbook of clinical chemistry and molecular diagnostics. 4th ed.Philadelphia: Elsevier Saunders;2006. p. 818–26.2. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003; 139:137–47.
Article3. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002; 39(S1):S1–266.4. Le Bricon T, Thervet E, Froissart M, Benlakehal M, Bousquet B, Legendre C, et al. Plasma cystatin C is superior to 24-h creatinine clearance and plasma creatinine for estimation of glomerular filtration rate 3 months after kidney transplantation. Clin Chem. 2000; 46:1206–7.
Article5. Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003; 41:1–12.
Article6. Mussap M, Plebani M. Biochemistry and clinical role of human cystatin C. Crit Rev Clin Lab Sci. 2004; 41:467–550.
Article7. Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO, et al. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995; 47:312–8.
Article8. Cataldi L, Mussap M, Bertelli L, Ruzzante N, Fanos V, Plebani M. Cystatin C in healthy women at term pregnancy and in their infant newborns?: relationship between maternal and neonatal serum levels and reference values. Am J Perinatal. 1999; 16:287–95.
Article9. Fischbach M, Graff V, Terzic J, Bergere V, Oudet M, Hamel G. Impact of age on reference values for serum concentration of cystatin C in children. Pediatr Nephrol. 2002; 17:104–6.
Article10. Norlund L, Fex G, Lanke J, Von Schenck H, Nilsson JE, Leksell H, et al. Reference intervals for the glomerular filtration rate and cell-proliferation markers: serum cystatin C and serum beta 2-microglo-bulin/cystain C-ratio. Scand J Clin Lab Invest. 1997; 57:463–70.11. Finney H, Newman DJ, Price CP. Adult reference ranges serum cystatin C, creatinine and predicted creatinine clearance. Ann Clin Biochem. 2000; 37:49–59.12. Erlandsen EJ, Randers E, Kristensen JH. Reference intervals for serum cystatin C and serum creatinine in adults. Clin Chem Lab Med. 1998; 36:393–7.
Article13. Uhlmann EJ, Hock KG, Issitt C, Sneeringer MR, Cervelli DR, Gorman RT, et al. Reference intervals for plasma cystatin C in healthy volunteers and renal patients, as measured by the Dade Behring BN II System, and correlation with creatinine. Clin Chem. 2001; 47:2031–3.
Article14. Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005; 67:2089–100.
Article15. Wiesli P, Schwegler B, Spinas GA, Schmid C. Serum cystatin C is sensitive to small changes in thyroid function. Clin Chim Acta. 2003; 338:87–90.
Article16. den Hollander JG, Wulkan RW, Mantel MJ, Berghout A. Is cystatin C a marker of glomerular filtration rate in thyroid dysfunction? Clin Chem. 2003; 49:1558–9.
Article17. Fricker M, Wiesli P, Brandle M, Schwegler B, Schmid C. Impact of thyroid dysfunction on serum cystatin C. Kidney Int. 2003; 63:1944–7.
Article18. Risch L, Herklotz R, Blumberg A, Huber AR. Effects of glucocorticoid immunosuppression on serum cystatin C concentrations in renal transplant patients. Clin Chem. 2001; 47:2055–9.
Article19. Tokyol C, Koken T, Demirbas M, Dilek FH, Yorukoglu K, Mungan U, et al. Expression of cathepsin D in bladder carcinoma: correlation with pathological features and serum cystatin C levels. Tumori. 2006; 92:230–5.
Article20. Saleh Y, Sebzda T, Warwas M, Kopec W, Ziolkowska J, Siewinski M. Expression of cystatin C in clinical human colorectal cancer tissues. J Exp Ther Oncol. 2005; 5:49–53.21. Demirtas S, Akan O, Can M, Elmali E, Akan H. Cystatin C can be affected by nonrenal factors: a preliminary study on leukemia. Clin Biochem. 2006; 39:115–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Relationship between serum cystatin C and glomerular filtration rate in renal transplant patients
- Evaluation of Glomerular Filtration Rate by Prediction Equation in the Elderly
- Comparison of serum cystatin C and creatinine as a marker for early detection of decreasing glomerular filtration rate in renal transplants
- Comparison of various methods of glomerular filtration rate measurements in children
- Evaluation of Serum Cystatin C Concentration as a Marker of Renal Function in Liver Cirrhosis Patients