Kawasaki Disease: Laboratory Findings and an Immunopathogenesis on the Premise of a "Protein Homeostasis System"
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea. leekyungyil@catholic.ac.kr
- KMID: 1120191
- DOI: http://doi.org/10.3349/ymj.2012.53.2.262
Abstract
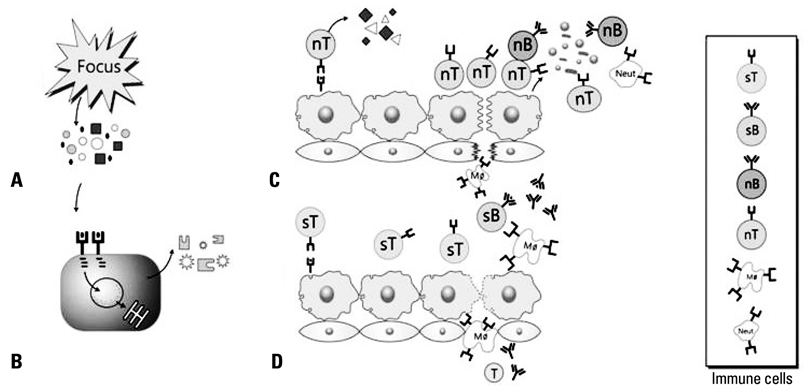
- Kawasaki disease (KD) is a self-limited systemic inflammatory illness, and coronary artery lesions (CALs) are a major complication determining the prognosis of the disease. Epidemiologic studies in Asian children suggest that the etiologic agent(s) of KD may be associated with environmental changes. Laboratory findings are useful for the diagnosis of incomplete KD, and they can guide the next-step in treatment of initial intravenous immunoglobulin non-responders. CALs seem to develop in the early stages of the disease before a peak in inflammation. Therefore early treatment, before the peak in inflammation, is mandatory to reduce the risk of CAL progression and severity of CALs. The immunopathogenesis of KD is more likely that of acute rheumatic fever than scarlet fever. A hypothetical pathogenesis of KD is proposed under the premise of a "protein homeostasis system"; where innate and adaptive immune cells control pathogenic proteins that are toxic to host cells at a molecular level. After an infection of unknown KD pathogen(s), the pathogenic proteins produced from an unknown focus, spread and bind to endothelial cells of coronary arteries as main target cells. To control the action of pathogenic proteins and/or substances from the injured cells, immune cells are activated. Initially, non-specific T cells and non-specific antibodies are involved in this reaction, while hyperactivated immune cells produce various cytokines, leading to a cytokine imbalance associated with further endothelial cell injury. After the emergence of specific T cells and specific antibodies against the pathogenic proteins, tissue injury ceases and a repair reaction begins with the immune cells.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
Inflammatory Cytokine and Osmolarity Changes in the Tears of Dry Eye Patients Treated with Topical 1% Methylprednisolone
Ji Hwan Lee, Kyung Min, Se Kyung Kim, Eung Kweon Kim, Tae-im Kim
Yonsei Med J. 2014;55(1):203-208. doi: 10.3349/ymj.2014.55.1.203.Severe Skin Lesions or Arthritis May be Associated with Coronary Artery Lesions in Kawasaki Disease
Song Ee Youn, Hee Young Ju, Kyung Suk Lee, Sung Ho Cha, Mi Young Han, Kyung Lim Yoon
Pediatr Infect Vaccine. 2016;23(2):102-108. doi: 10.14776/piv.2016.23.2.102.A Common Immunopathogenesis Mechanism for Infectious Diseases: The Protein-Homeostasis-System Hypothesis
Kyung-Yil Lee
Infect Chemother. 2015;47(1):12-26. doi: 10.3947/ic.2015.47.1.12.Clinical Features of Kawasaki Disease with Pyuria
Hyo-Jin Kim, Joo-Young Lee, Ui-Yoon Choi, Soo-Young Lee
Pediatr Infect Vaccine. 2017;24(3):141-145. doi: 10.14776/piv.2017.24.3.141.
Reference
-
1. Kawasaki T. [Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children]. Arerugi. 1967. 16:178–222.2. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004. 114:1708–1733.
Article3. Kato H, Sugimura T, Akagi T, Sato N, Hashino K, Maeno Y, et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation. 1996. 94:1379–1385.4. Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991. 324:1633–1639.
Article5. Fujiwara H, Hamashima Y. Pathology of the heart in Kawasaki disease. Pediatrics. 1978. 61:100–107.
Article6. Amano S, Hazama F, Kubagawa H, Tasaka K, Haebara H, Hamashima Y. General pathology of Kawasaki disease. On the morphological alterations corresponding to the clinical manifestations. Acta Pathol Jpn. 1980. 30:681–694.7. Brown TJ, Crawford SE, Cornwall ML, Garcia F, Shulman ST, Rowley AH. CD8 T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J Infect Dis. 2001. 184:940–943.
Article8. Rowley AH, Shulman ST, Mask CA, Finn LS, Terai M, Baker SC, et al. IgA plasma cell infiltration of proximal respiratory tract, pancreas, kidney, and coronary artery in acute Kawasaki disease. J Infect Dis. 2000. 182:1183–1191.
Article9. Choi IH, Chwae YJ, Shim WS, Kim DS, Kwon DH, Kim JD, et al. Clonal expansion of CD8+ T cells in Kawasaki disease. J Immunol. 1997. 159:481–486.10. Furuno K, Yuge T, Kusuhara K, Takada H, Nishio H, Khajoee V, et al. CD25+CD4+ regulatory T cells in patients with Kawasaki disease. J Pediatr. 2004. 145:385–390.
Article11. Galeotti C, Bayry J, Kone-Paut I, Kaveri SV. Kawasaki disease: aetiopathogenesis and therapeutic utility of intravenous immunoglobulin. Autoimmun Rev. 2010. 9:441–448.
Article12. Kim DS. Kawasaki disease. Yonsei Med J. 2006. 47:759–772.
Article13. Lee KY, Han JW, Lee JS. Kawasaki disease may be a hyperimmune reaction of genetically susceptible children to variants of normal environmental flora. Med Hypotheses. 2007. 69:642–651.
Article14. Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004. 364:533–544.
Article15. Yeung RS. Kawasaki disease: update on pathogenesis. Curr Opin Rheumatol. 2010. 22:551–560.
Article16. Rowley AH, Shulman ST. Pathogenesis and management of Kawasaki disease. Expert Rev Anti Infect Ther. 2010. 8:197–203.
Article17. Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. 2006. 149:237–240.
Article18. Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006. 113:2606–2612.
Article19. Park YW, Han JW, Hong YM, Ma JS, Cha SH, Kwon TC, et al. Epidemiological features of Kawasaki disease in Korea, 2006-2008. Pediatr Int. 2011. 53:36–39.
Article20. Nakamura Y, Yashiro M, Uehara R, Sadakane A, Chihara I, Aoyama Y, et al. Epidemiologic features of Kawasaki disease in Japan: results of the 2007-2008 nationwide survey. J Epidemiol. 2010. 20:302–307.
Article21. Huang WC, Huang LM, Chang IS, Chang LY, Chiang BL, Chen PJ, et al. Epidemiologic features of Kawasaki disease in Taiwan, 2003-2006. Pediatrics. 2009. 123:e401–e405.
Article22. Kushner HI, Macnee RP, Burns JC. Kawasaki disease in India: increasing awareness or increased incidence? Perspect Biol Med. 2009. 52:17–29.
Article23. Kim SH, Kim KH, Kim DS. Clinical characteristics of Kawasaki disease according to age at diagnosis. Indian Pediatr. 2009. 46:585–590.24. Yeung RS. Phenotype and coronary outcome in Kawasaki's disease. Lancet. 2007. 369:85–87.
Article25. Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. 2008. 153:117–121.
Article26. Youn YS, Lee KY, Hwang JY, Rhim JW, Kang JH, Lee JS, et al. Difference of clinical features in childhood Mycoplasma pneumoniae pneumonia. BMC Pediatr. 2010. 10:48.
Article27. Li AM, Ng PC. Severe acute respiratory syndrome (SARS) in neonates and children. Arch Dis Child Fetal Neonatal Ed. 2005. 90:F461–F465.
Article28. Dentinger CM. Emerging infections: hepatitis A. Am J Nurs. 2009. 109:29–33.
Article29. Rhim JW, Lee KY, Youn YS, Kang JH, Kim JC. Epidemiological and clinical characteristics of childhood pandemic 2009 H1N1 virus infection: an observational cohort study. BMC Infect Dis. 2011. 11:225.
Article30. Powell DA, Hunt WG. Tuberculosis in children: an update. Adv Pediatr. 2006. 53:279–322.
Article31. Sheehy SH, Angus BJ. Malaria: severe, life-threatening. Clin Evid (Online). 2011. pii: 0913.32. Lee KY, Hong JH, Han JW, Lee JS, Lee BC, Burgner D. Features of Kawasaki disease at the extremes of age. J Paediatr Child Health. 2006. 42:423–427.
Article33. Pannaraj PS, Turner CL, Bastian JF, Burns JC. Failure to diagnose Kawasaki disease at the extremes of the pediatric age range. Pediatr Infect Dis J. 2004. 23:789–791.
Article34. Holman RC, Curns AT, Belay ED, Steiner CA, Schonberger LB. Kawasaki syndrome hospitalizations in the United States, 1997 and 2000. Pediatrics. 2003. 112:495–501.
Article35. Holman RC, Curns AT, Belay ED, Steiner CA, Effler PV, Yorita KL, et al. Kawasaki syndrome in Hawaii. Pediatr Infect Dis J. 2005. 24:429–433.
Article36. Onouchi Y. Molecular genetics of Kawasaki disease. Pediatr Res. 2009. 65:46R–54R.
Article37. Onouchi Y, Tamari M, Takahashi A, Tsunoda T, Yashiro M, Nakamura Y, et al. A genomewide linkage analysis of Kawasaki disease: evidence for linkage to chromosome 12. J Hum Genet. 2007. 52:179–190.
Article38. Burgner D, Davila S, Breunis WB, Ng SB, Li Y, Bonnard C, et al. A genome-wide association study identifies novel and functionally related susceptibility loci for Kawasaki disease. PLoS Genet. 2009. 5:e1000319.
Article39. Chi H, Huang FY, Chen MR, Chiu NC, Lee HC, Lin SP, et al. ITPKC gene SNP rs28493229 and Kawasaki disease in Taiwanese children. Hum Mol Genet. 2010. 19:1147–1151.
Article40. Kim JJ, Hong YM, Sohn S, Jang GY, Ha KS, Yun SW, et al. A genome-wide association analysis reveals 1p31 and 2p13.3 as susceptibility loci for Kawasaki disease. Hum Genet. 2011. 129:487–495.
Article41. Burns JC. Kawasaki Disease update. Indian J Pediatr. 2009. 76:71–76.
Article42. Onouchi Y, Gunji T, Burns JC, Shimizu C, Newburger JW, Yashiro M, et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. 2008. 40:35–42.
Article43. Kuo HC, Yang KD, Juo SH, Liang CD, Chen WC, Wang YS, et al. ITPKC single nucleotide polymorphism associated with the Kawasaki disease in a Taiwanese population. PLoS One. 2011. 6:e17370.
Article44. Kato H, Fujimoto T, Inoue O, Kondo M, Koga Y, Yamamoto S, et al. Variant strain of Propionibacterium acnes: a clue to the aetiology of Kawasaki disease. Lancet. 1983. 2:1383–1388.
Article45. Shinomiya N, Takeda T, Kuratsuji T, Takagi K, Kosaka T, Tatsuzawa O, et al. Variant Streptococcus sanguis as an etiological agent of Kawasaki disease. Prog Clin Biol Res. 1987. 250:571–572.46. Abe J, Kotzin BL, Jujo K, Melish ME, Glode MP, Kohsaka T, et al. Selective expansion of T cells expressing T-cell receptor variable regions V beta 2 and V beta 8 in Kawasaki disease. Proc Natl Acad Sci U S A. 1992. 89:4066–4070.
Article47. Iwanaga M, Takada K, Osato T, Saeki Y, Noro S, Sakurada N. Kawasaki disease and Epstein-Barr virus. Lancet. 1981. 1:938–939.
Article48. Burns JC, Geha RS, Schneeberger EE, Newburger JW, Rosen FS, Glezen LS, et al. Polymerase activity in lymphocyte culture supernatants from patients with Kawasaki disease. Nature. 1986. 323:814–816.
Article49. Esper F, Shapiro ED, Weibel C, Ferguson D, Landry ML, Kahn JS. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis. 2005. 191:499–502.
Article50. Lidar M, Lipschitz N, Langevitz P, Shoenfeld Y. The infectious etiology of vasculitis. Autoimmunity. 2009. 42:432–438.
Article51. Sepp E, Julge K, Vasar M, Naaber P, Björksten B, Mikelsaar M. Intestinal microflora of Estonian and Swedish infants. Acta Paediatr. 1997. 86:956–961.
Article52. Adlerberth I, Carlsson B, de Man P, Jalil F, Khan SR, Larsson P, et al. Intestinal colonization with Enterobacteriaceae in Pakistani and Swedish hospital-delivered infants. Acta Paediatr Scand. 1991. 80:602–610.
Article53. Furusho K, Kamiya T, Nakano H, Kiyosawa N, Shinomiya K, Hayashidera T, et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1984. 2:1055–1058.
Article54. Hicks RV, Melish ME. Kawasaki syndrome; Rheumatic complains and analysis of salicylate therapy. Arthritis Rheum. 1979. 22:621–622.55. Lee KY, Han JW, Hong JH, Lee HS, Lee JS, Whang KT. Inflammatory processes in Kawasaki disease reach their peak at the sixth day of fever onset: laboratory profiles according to duration of fever. J Korean Med Sci. 2004. 19:501–504.
Article56. Newburger JW, Sleeper LA, McCrindle BW, Minich LL, Gersony W, Vetter VL, et al. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N Engl J Med. 2007. 356:663–675.
Article57. Harada K. Intravenous gamma-globulin treatment in Kawasaki disease. Acta Paediatr Jpn. 1991. 33:805–810.58. Lee KY, Han JW, Lee HS, Hong JH, Hahn SH, Lee JS, et al. Epidemiologic study of Kawasaki disease at a single hospital in Daejeon, Korea (1987 through 2000). Pediatr Infect Dis J. 2004. 23:52–55.
Article59. Mori M, Imagawa T, Yasui K, Kanaya A, Yokota S. Predictors of coronary artery lesions after intravenous gamma-globulin treatment in Kawasaki disease. J Pediatr. 2000. 137:177–180.
Article60. Fukunishi M, Kikkawa M, Hamana K, Onodera T, Matsuzaki K, Matsumoto Y, et al. Prediction of non-responsiveness to intravenous high-dose gamma-globulin therapy in patients with Kawasaki disease at onset. J Pediatr. 2000. 137:172–176.
Article61. Durongpisitkul K, Soongswang J, Laohaprasitiporn D, Nana A, Prachuabmoh C, Kangkagate C. Immunoglobulin failure and retreatment in Kawasaki disease. Pediatr Cardiol. 2003. 24:145–148.
Article62. Sano T, Kurotobi S, Matsuzaki K, Yamamoto T, Maki I, Miki K, et al. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr. 2007. 166:131–137.
Article63. Uehara R, Belay ED, Maddox RA, Holman RC, Nakamura Y, Yashiro M, et al. Analysis of potential risk factors associated with nonresponse to initial intravenous immunoglobulin treatment among Kawasaki disease patients in Japan. Pediatr Infect Dis J. 2008. 27:155–160.
Article64. Kim SK, Han JY, Rhim JW, Oh JH, Han JW, Lee KY, et al. Limitation of prediction on intravenous immunoglobulin responsiveness in Kawasaki disease. Korean J Pediatr Infect Dis. 2010. 17:169–176.
Article65. Kelley-Hedgepeth A, Lloyd-Jones DM, Colvin A, Matthews KA, Johnston J, Sowers MR, et al. Ethnic differences in C-reactive protein concentrations. Clin Chem. 2008. 54:1027–1037.
Article66. Jibiki T, Terai M, Kurosaki T, Nakajima H, Suzuki K, Inomata H, et al. Efficacy of intravenous immune globulin therapy combined with dexamethasone for the initial treatment of acute Kawasaki disease. Eur J Pediatr. 2004. 163:229–233.
Article67. Inoue Y, Okada Y, Shinohara M, Kobayashi T, Kobayashi T, Tomomasa T, et al. A multicenter prospective randomized trial of corticosteroids in primary therapy for Kawasaki disease: clinical course and coronary artery outcome. J Pediatr. 2006. 149:336–341.
Article68. Yeo JS, Choi JW. Effectiveness of medium-dose intravenous immunoglobulin (1 g/kg) in the treatment of Kawasaki disease. Korean Circ J. 2010. 40:81–85.
Article69. Terai M, Shulman ST. Prevalence of coronary artery abnormalities in Kawasaki disease is highly dependent on gamma globulin dose but independent of salicylate dose. J Pediatr. 1997. 131:888–893.
Article70. Lee KY, Lee HS, Hong JH, Han JW, Lee JS, Whang KT. High-dose intravenous immunoglobulin downregulates the activated levels of inflammatory indices except erythrocyte sedimentation rate in acute stage of Kawasaki Disease. J Trop Pediatr. 2005. 51:98–101.
Article71. Lee KY, Han JW, Lee JS, Whang KT. Alteration of biochemical profiles after high-dose intravenous immunoglobulin administration in Kawasaki disease. Acta Paediatr. 2002. 91:164–167.
Article72. Lee KY, Kim DU, Lee HS, Jang PS, Kim YH, Kim JT, et al. The effects of high-dose intravenous immunoglobulin on plasma protein and lipid levels in the patients with Kawasaki disease. Korean J Pediatr. 2006. 49:1348–1353.
Article73. Lee KY, Lee JS. Immunoglobulin G has a role for systemic protein modulation in vivo: a new concept of protein homeostasis. Med Hypotheses. 2006. 67:848–855.
Article74. Terai M, Honda T, Yasukawa K, Higashi K, Hamada H, Kohno Y. Prognostic impact of vascular leakage in acute Kawasaki disease. Circulation. 2003. 108:325–330.
Article75. Hwang JY, Lee KY, Rhim JW, Youn YS, Oh JH, Han JW, et al. Assessment of intravenous immunoglobulin non-responders in Kawasaki disease. Arch Dis Child. 2011. 96:1088–1090.
Article76. Sundel RP, Burns JC, Baker A, Beiser AS, Newburger JW. Gamma globulin re-treatment in Kawasaki disease. J Pediatr. 1993. 123:657–659.
Article77. Freeman AF, Shulman ST. Refractory Kawasaki disease. Pediatr Infect Dis J. 2004. 23:463–464.
Article78. Wallace CA, French JW, Kahn SJ, Sherry DD. Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics. 2000. 105:E78.
Article79. Burns JC, Best BM, Mejias A, Mahony L, Fixler DE, Jafri HS, et al. Infliximab treatment of intravenous immunoglobulin-resistant Kawasaki disease. J Pediatr. 2008. 153:833–838.
Article80. Wright DA, Newburger JW, Baker A, Sundel RP. Treatment of immune globulin-resistant Kawasaki disease with pulsed doses of corticosteroids. J Pediatr. 1996. 128:146–149.
Article81. Lee TJ, Kim KH, Chun JK, Kim DS. Low-dose methotrexate therapy for intravenous immunoglobulin-resistant Kawasaki disease. Yonsei Med J. 2008. 49:714–718.
Article82. Mori M, Imagawa T, Katakura S, Miyamae T, Okuyama K, Ito S, et al. Efficacy of plasma exchange therapy for Kawasaki disease intractable to intravenous gamma-globulin. Mod Rheumatol. 2004. 14:43–47.
Article83. Shulman ST, Tanz RR. Group A streptococcal pharyngitis and immune-mediated complications: from diagnosis to management. Expert Rev Anti Infect Ther. 2010. 8:137–150.
Article84. Lappin E, Ferguson AJ. Gram-positive toxic shock syndromes. Lancet Infect Dis. 2009. 9:281–290.
Article85. Olsen RJ, Shelburne SA, Musser JM. Molecular mechanisms underlying group A streptococcal pathogenesis. Cell Microbiol. 2009. 11:1–12.
Article86. Bryant PA, Robins-Browne R, Carapetis JR, Curtis N. Some of the people, some of the time: susceptibility to acute rheumatic fever. Circulation. 2009. 119:742–753.87. Guilherme L, Kalil J. Rheumatic fever and rheumatic heart disease: cellular mechanisms leading autoimmune reactivity and disease. J Clin Immunol. 2010. 30:17–23.
Article88. Lloyd S, Mead S, Collinge J. Genetics of prion disease. Top Curr Chem. 2011. 305:1–22.
Article89. Matzinger P. The danger model: a renewed sense of self. Science. 2002. 296:301–305.
Article90. Tveita AA. The danger model in deciphering autoimmunity. Rheumatology (Oxford). 2010. 49:632–639.
Article91. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009. 22:240–273.
Article92. Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011. 30:16–34.
Article93. Schneider F, Tomek W, Gründker C. Gonadotropin-releasing hormone (GnRH) and its natural analogues: a review. Theriogenology. 2006. 66:691–709.
Article94. Shruti K, Shrey K, Vibha R. Micro RNAs: tiny sequences with enormous potential. Biochem Biophys Res Commun. 2011. 407:445–449.
Article95. Macias ES, Pereira FA, Rietkerk W, Safai B. Superantigens in dermatology. J Am Acad Dermatol. 2011. 64:455–472.
Article96. Bran GM, Goessler UR, Hormann K, Riedel F, Sadick H. Keloids: current concepts of pathogenesis (review). Int J Mol Med. 2009. 24:283–293.
Article97. Mucida D, Cheroutre H. The many face-lifts of CD4 T helper cells. Adv Immunol. 2010. 107:139–152.
Article98. Lee KY. Pediatric respiratory infections by Mycoplasma pneumoniae. Expert Rev Anti Infect Ther. 2008. 6:509–521.99. Lee KY, Rhim JW, Kang JH. Hyperactive immune cells (T cells) may be responsible for acute lung injury in influenza virus infections: a need for early immune-modulators for severe cases. Med Hypotheses. 2011. 76:64–69.
Article100. Kil HR, Lee JH, Lee KY, Rhim JW, Youn YS, Kang JH. Early corticosteroid treatment for severe pneumonia caused by 2009 H1N1 influenza virus. Crit Care. 2011. 15:413.
Article101. Lehman TJ, Warren R, Gietl D, Mahnovski V, Prescott M. Variable expression of Lactobacillus casei cell wall-induced coronary arteritis: an animal model of Kawasaki's disease in selected inbred mouse strains. Clin Immunol Immunopathol. 1988. 48:108–118.
Article102. Schulte DJ, Yilmaz A, Shimada K, Fishbein MC, Lowe EL, Chen S, et al. Involvement of innate and adaptive immunity in a murine model of coronary arteritis mimicking Kawasaki disease. J Immunol. 2009. 183:5311–5318.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Common Immunopathogenesis Mechanism for Infectious Diseases: The Protein-Homeostasis-System Hypothesis
- Immunopathogenesis of Kawasaki Disease
- Kawasaki Disease
- Immunopathogenesis of COVID-19 and early immunomodulators
- Etiological and pathophysiological enigmas of severe coronavirus disease 2019, multisystem inflammatory syndrome in children, and Kawasaki disease