Yonsei Med J.
2006 Apr;47(2):223-229. 10.3349/ymj.2006.47.2.223.
Generation of Nitric Oxide in the Opossum Lower Esophageal Sphincter during Physiological Experimentation
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. hjpark21@yumc.yonsei.ac.kr
- 2Department of Internal Medicine. Cedars-Sinai Medical Center, Los Angeles, CA, USA.
- KMID: 1110746
- DOI: http://doi.org/10.3349/ymj.2006.47.2.223
Abstract
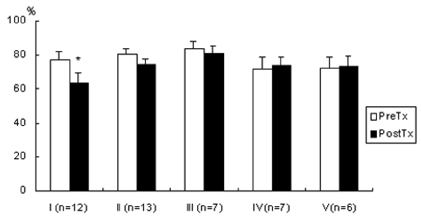
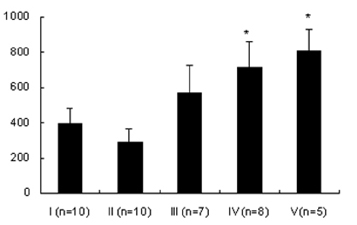
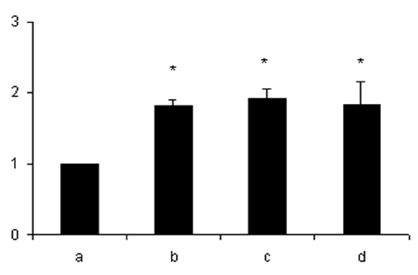
- Lipopolysaccharide (LPS), given in vivo, modulates opossum esophageal motor functions by inducing the inducible nitric oxide synthase (iNOS), which increases nitric oxide (NO) production. Superoxide, a NO scavenger, is generated during this endotoxemia. Superoxide is cleared by superoxide dismutase (SOD) and catalase (CAT) to protect the physiological function of NO. This study examined whether lower esophageal sphincter (LES) motility, NO release, and iNOS and nitrotyrosine accumulation in the LES are affected by LPS in vitro. Muscle strips from the opossum LES were placed in tissue baths containing oxygenated Krebs buffer. NO release was measured with a chemiluminescence NOx analyzer, and Western blots were performed to analyze iNOS and nitrotyrosine production. The percent change in resting LES tone after a 6-hour exposure to LPS was significantly increased compared to pretreatment values. The percent LES relaxation upon electrical stimulation was significantly decreased in the control group at 6 hours, indicating that the LPS treatment had an effect. The NO concentration in the tissue bath of LPS-treated muscle without nerve stimulation was significantly less than that of LPS treatment combined with SOD/CAT or SOD/CAT alone. iNOS and nitrotyrosine were detectable and increased over time in the LES muscle of both the control and LPS-treated groups. Antioxidant enzymes may play a role in regulating NO-mediated neuromuscular functions in the LES.
Keyword
MeSH Terms
-
Tyrosine/analogs & derivatives/chemistry
Time Factors
Superoxide Dismutase/metabolism
Opossums
Nitric Oxide Synthase Type II/metabolism
Nitric Oxide/*chemistry/metabolism
Muscles/metabolism
Male
Luminescence
Lipopolysaccharides/chemistry/metabolism
Female
Esophageal Sphincter, Upper/*anatomy & histology/metabolism
Esophageal Sphincter, Lower/*anatomy & histology/metabolism
Catalase/metabolism
Blotting, Western
Antioxidants/chemistry/metabolism
Animals
Figure
Reference
-
1. Park H, Cullen JJ, Clark E, Conklin JL. Effect of inhibiting iNOS on endotoxin-induced changes in esophageal motor function. Neurogastroenterol Motil. 1999. 11:278A.2. Park H, Clark E, Cullen JJ, Conklin JL. Effect of endotoxin on opossum esophageal motor function. Neurogastroenterol Motil. 2000. 12:215–221.3. Brovkovych V, Patton S, Brovkovych S, Kiechle F, Huk I, Malinski T. In situ measurement of nitric oxide, superoxide and peroxynitrite during endotoxemia. J Physiol Pharmacol. 1997. 48:633–644.4. Leichus LS, Thomas RM, Murray JA, Conklin JL. Effect of oxygen radicals and radical scavenging on opossum lower esophageal sphincter. Dig Dis Sci. 1997. 42:592–596.5. Thomas RM, Fang S, Leichus LS, Oberley LW, Christensen J, Murray JA, et al. Antioxidant enzymes in intramural nerves of the opossum esophagus. Am J Physiol. 1996. 270(1 Pt 1):G136–G142.6. Arkovitz MS, Wispe JR, Garcia VF, Szabo C. Selective inhibition of the inducible isoform of nitric oxide synthase prevents pulmonary transvascular flux during acute endotoxemia. J Pediatr Surg. 1996. 31:1009–1015.7. Hamilton CA, Berg G, Mcintyre M, Mcphaden AR, Reid JL, Dominiczak AF. Effects of nitric oxide and superoxide on relaxation in human artery and vein. Atherosclerosis. 1997. 133:77–86.8. Muijsers RB, Folkerts G, Henricks PA, Sadeghi-Hashjin G, Nijkamp FP. Peroxynitrite: a two-faced metabolite of nitric oxide. Life Sci. 1997. 60:1833–1845.9. Miller MJ, Thompson JH, Zhang XJ, Sadowska-Krowicka H, Kakkis JL, Munshi UK, et al. Role of inducible nitric oxide synthase expression and peroxynitrite formation in guinea pig ileitis. Gastroenterology. 1995. 109:1475–1483.10. Flesch M, Kilter H, Cremers B, Laufs U, Sudkamp M, Ortmann M, et al. Effects of endotoxin on human myocardial contractility involvement of nitric oxide and peroxynitrite. J Am Coll Cardiol. 1999. 33:1062–1070.11. Takakura K, Goto Y, Muramatsu I. Nitric oxide synthase induction and relaxation in lipopolysaccharide-treated gastric fundus muscle of rats. Life Sci. 1996. 58:9–17.12. Brune B, Schmidt KU, Ullrich V. Activation of soluble guanylate cyclase by carbon monoxide and inhibition by superoxide anion. Eur J Bioochem. 1990. 192:683–688.13. Murray JA, Du C, Ledlow A, Manternach PL, Conklin JL. Guanyl cyclase inhibitors: effect on tone, relaxation and cGMP content of the lower esophageal sphincter. Am J Physiol. 1992. 263:G97–G101.14. Uc A, Murray JA, Kooy N, Conklin JL. Effect of peroxynitrite on motor functions of the opossum esophagus. Dig Dis Sci. 2001. 46:30–37.15. Boota A, Zar H, Kim YM, Johnson B, Pitt B, Davies P. IL-1 stimulates superoxide and delayed peroxynitrite production by pulmonary vascular smooth muscle cells. Am J Physiol. 1996. 271(6 Pt 1):L932–L938.16. Yang CC, Alvarez RB, Engel WK, Askanas V. Increase of nitric oxide synthases and nitrotyrosine in inclusion-body myositis. Neuroreport. 1996. 8:153–158.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Medical and Endoscopic Management of Achalasia
- Bacterial Lipopolysaccharide - Induced Generation of Nitric Oxide is Mediated by ERK1 and JNK1 / SAPK in Primary Rat Neonatal Astrocytes
- The Potential Role of Nitric Oxide in Halting Cancer Progression Through Chemoprevention
- The Study of neuronal Nitric Oxide Synthase (nNOS) Gene Polymorphism in Primary Esophageal Motility Disorders
- Nitric Oxide in the Kidney: Its Physiological Role and Pathophysiological Implications