Yonsei Med J.
2006 Apr;47(2):179-183. 10.3349/ymj.2006.47.2.179.
A Comparative Study of Magnetic-Activated Cell Sorting, Cytotoxicity and Preplating for the Purification of Human Myoblasts
- Affiliations
-
- 1Department of Rehabilitation Medicine and Rehabilitation Institute of Muscular Disease, Yonsei University College of Medicine, Seoul, Korea. drtlc@yumc.yonsei.ac.kr
- KMID: 1110739
- DOI: http://doi.org/10.3349/ymj.2006.47.2.179
Abstract
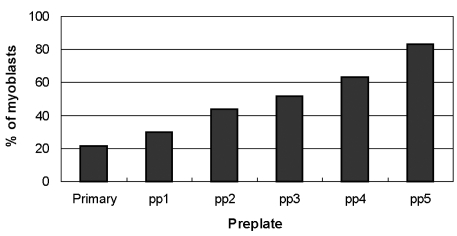
- Although cultured myoblast transplantation has been extensively studied as a gene complementation approach to muscular dystrophy treatment, clinical success has still been limited. The inability to adequately isolate and purify myoblasts presents a major limitation to the production of sufficient myoblasts for engrafting purposes. This study attempted to purify myoblasts from primary culture by magnetic-activated cell sorting (MACS), complement-mediated cytotoxicity, and a preplating technique. As a result of positive myoblasts selection by MACS, the average percentage of myoblasts in mixed culture was increased from 30.0% to 41.7%. We observed both myoblast lysis and fibroblast lysis after complement-mediated cytotoxicity. Enrichment of myoblasts in mixed culture was found to increase to 83.1% by using the preplating technique. In addition, higher purification (92.8%) was achieved by following the preplating technique with MACS. Thus, preplating in combination with magnetic-activated cell sorting allows for a rapid and effective isolation of myoblasts from human muscle tissue.
Keyword
MeSH Terms
Figure
Reference
-
1. Arahata K, Ishiura S, Ishiguro T, Tsukahara T, Suhara Y, Eguchi C, et al. Immunostaining of skeletal and cardiac muscle surface membrane with antibody against Duchenne muscular dystrophy peptide. Nature. 1988; 333:861–863. PMID: 3290683.
Article2. Hoffman EP, Brown J, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987; 51:919–928. PMID: 3319190.
Article3. Huard J, Labrecque C, Dansereau G, Robitaille L, Tremblay JP, Labrecque C, et al. Dystrophin expression in myotubes formed by the fusion of normal and dystrophic myoblasts. Muscle Nerve. 1991; 14:178–182. PMID: 2000106.
Article4. Monaco AP, Neve RL, Colletti-Feener C, Bertlson CJ, Kurnit DM, Kunkel LM. Isolation of candidate cDNAs for portion of the Duchenne muscular dystrophy gene. Nature. 1986; 323:646–650. PMID: 3773991.5. Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibers from dystrophin negative to positive by injection of normal myoblasts. Nature. 1989; 337:176–179. PMID: 2643055.6. Thomas MA, Fast A, Bach JR. DeLisa JA, Gans BM, editors. Rehabilitation of the patient with disease of the motor unit. Rehabilitation medicine: principles and practice. 1993. Philadelphia: Lippincott Company;p. 1564–1568.7. Zubrzycka-Gaarn EE, Bulman DE, Karpati G, Burghes AH, Belfall B, Klamut HJ, et al. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature. 1988; 333:466–469. PMID: 3287171.
Article8. Morgan JE, Hoffman EP, Partridge TA. Normal myogenic cells from newborn mice restore normal histology to degenerating muscle of the mdx mouse. J Cell Biol. 1990; 111:2437–2449. PMID: 2277066.9. Watt DJ, Lambert K, Morgan JE, Partridge TA, Sloper JC. Incorporation of donor muscle precursor cells into an area of muscle regeneration in the host mouse. J Neurol Sci. 1982; 57:319–331. PMID: 6761411.
Article10. Watt DJ, Morgan JE, Partridge TA. Use of mononuclear precursor cells to insert allogeneic genes into growing mouse muscles. Muscle Nerve. 1984; 7:741–750. PMID: 6599303.
Article11. Chiu RC, Zibaitis A, Kao RL. Cellular cardiomyoplasty: myocardial regeneration with satellite cell implantation. Ann Thorac Surg. 1995; 60:12–18. PMID: 7598572.
Article12. Kao WW, Prockop DJ. Proline analogue removes fibroblasts from cultured mixed cell population. Nature. 1977; 266:63–64. PMID: 758007.13. Yablonka-Reuveni Z, Nameroff M. Skeletal muscle cell populations. Separation and partial characterization of fibroblast-like cells from embryonic tissue using density centrifugation. Histochemistry. 1987; 87:27–38. PMID: 3038797.14. Baroffio A, Aubry JP, Kaelin A, Krause RM, Hamann M, Bader CR. Purification of human muscle satellite cells by flow cytometry. Muscle Nerve. 1993; 16:498–505. PMID: 8515758.
Article15. Blanton JR Jr, Grant AL, McFarland DC, Robinson JP, Bidwell CA. Isolation of two populations of myoblasts from porcine skeletal muscle. Muscle Nerve. 1999; 22:43–50. PMID: 9883856.
Article16. Webster C, Pavlath GK, Parks DR, Walsh FS, Blau HM. Isolation of human myoblasts with the fluorescence-activated cell sorter. Exp Cell Res. 1988; 174:252–256. PMID: 3335226.
Article17. Qu Z, Balkir L, van Deutekom JC, Robbins PD, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998; 142:1257–1267. PMID: 9732286.
Article18. Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994; 125:1275–1287. PMID: 8207057.
Article19. Qu Z, Huard J. Matching host muscle and donor myoblasts for myosin heavy chain improves myoblast transfer therapy. Gene Therapy. 2000; 58:428–437. PMID: 10694825.
Article20. Lequerica JL, Mirabet V, Montero JA, Hurtado C, Piquer S, Carbonell F. In vitro proliferation, differentiation and immuno-magnetic bead purification of human myoblasts. Ann Transplant. 1999; 4:103–196. PMID: 10853794.21. Jankowski RJ, Haluszczak C, Trucco M, Huard J. Flow cytometric characterization of myogenic cell populations obtained via the preplate technique: potential for rapid isolation of muscle-derived stem cells. Hum Gene Ther. 2001; 12:619–628. PMID: 11426462.
Article22. Abboud CN, Duerst RE, Farntz CN, Ryan DH, Liesveld JL, Brennan JK. Lysis of human fibroblast colony-forming cells and endothelial cell by monoclonal antibody (6-9) and complement. Blood. 1986; 68:1196–1200. PMID: 3779098.23. Singer KH, Scearce RM, Tuck DT, Whichard LP, Denning SM, Haynes BF. Removal of fibroblasts from human epithelial cell cultures with use of a complement fixing monoclonal antibody reactive with human fibroblasts and monocytes/macrophages. J Invest Dermatol. 1989; 92:166–170. PMID: 2918230.
Article24. Simonart T, Degraef C, Heenen M, Hermans P, Van Vooren JP, Noel JC. Expression of the fibroblast/macrophage marker 1B10 by spindle cells in Kaposi's sarcoma lesions and by Kaposi's sarcoma-derived tumor cells. J Cutan Pathol. 2002; 29:72–78. PMID: 12150136.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- High efficiency transduction of human VEGF(165)into human skeletal myoblasts: in vitro studies
- Cytotoxicity of lymphokine activated peritoneal macrophages against Trichomonas vaginalis
- An experimental study on the cytotoxicity of recycled brackets
- Cytotoxicity of resident and lymphokine-activated mouse peritoneal macrophage against Trichomonas vaginalis
- IL-2-enhanced NK Cell Cytotoxicity is Regulated by Adiponectin from Hypothalamo-pituitary-adrenal Axis