J Vet Sci.
2008 Jun;9(2):155-160. 10.4142/jvs.2008.9.2.155.
Antigenic diversity of Theileria major piroplasm surface protein gene in Jeju black cattle
- Affiliations
-
- 1College of Veterinary Medicine, Cheju National University, Jeju 690-756, Korea. dvmyun@cheju.ac.kr
- 2Equine Center, Korea Racing Authority, Gwacheon 427-711, Korea.
- KMID: 1106230
- DOI: http://doi.org/10.4142/jvs.2008.9.2.155
Abstract
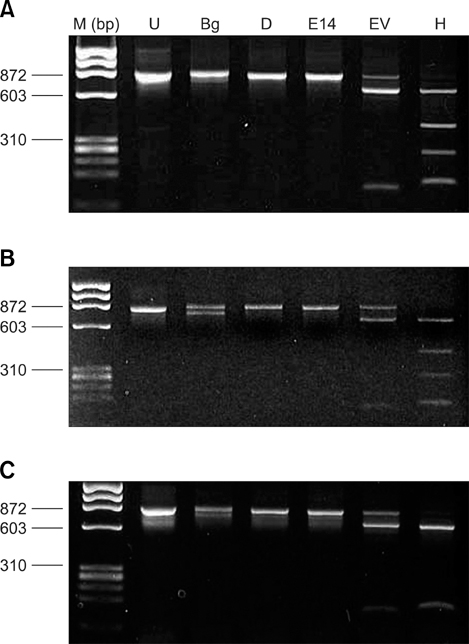
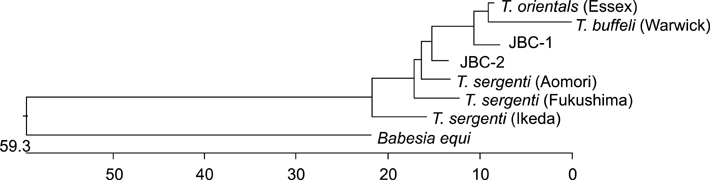
- Piroplasms are tick-transmitted, intracellular, hemoprotozoan parasites that cause anorexia, fever, anemia, and icterus. Theileriosis is caused by Theileria sergenti and causes major economic losses in grazing cattle in Japan and Korea. In May 2003, we examined the antigenic diversity of the major piroplasm surface protein (MPSP) gene in 35 healthy Jeju black cattle that were born and raised at the National Institute of Subtropical Agriculture. On microscopic examination of Giemsa-stained blood smears, 9 of 35 cattle had intra-erythrocytic piroplasms. Hematological data were within normal range for all 35 cattle. Amplification of DNA from all blood samples using universal MPSP gene primers showed mixed infections with C, I, and B type Theileria spp. Type C was identified in 20 of 35 blood samples, and type B was identified in 17 samples. Allelic variation was seen in type B.
Keyword
MeSH Terms
Figure
Reference
-
1. Chae JS, Lee JM, Kwon OD, Lee SO, Chae KS, Onuma M. Comparative analyses of Theileria sergenti isolated from Korea and Japan by Southern hybridization and polymerase chain reaction. Korean J Vet Res. 1996. 36:187–193.2. Cho SH, Kim TS, Lee HW, Tsuji M, Ishihara C, Kim JT, Wee SH, Lee CG. Identification of newly isolated Babesia parasites from cattle in Korea by using the Bo-RBC-SCID mice. Korean J Parasitol. 2002. 40:33–40.
Article3. Inoue M, Van Nguyen D, Meas S, Ohashi K, Sen S, Sugimoto C, Onuma M. Survey of Theileria parasite infection in cattle in Cambodia and Vietnam using piroplasm surface protein gene-specific polymerase chain reaction. J Vet Med Sci. 2001. 63:1155–1157.
Article4. Iwasaki T, Kakuda T, Sako Y, Sugimoto C, Onuma M. Differentiation and quantification of Theileria sergenti piroplasm types using type-specific monoclonal antibodies. J Vet Med Sci. 1998. 60:665–669.
Article5. Kakuda T, Kubota S, Sugimoto C, Baek BK, Yin H, Onuma M. Analysis of immunodominant piroplasm surface protein genes of benign Theileria parasites distributed in China and Korea by allele-specific polymerase chain reaction. J Vet Med Sci. 1998. 60:237–239.
Article6. Kang SW, Choi EJ, Kweon CH. Cloning and sequencing of p33 in a Korean isolate of Theileria sergenti. Korean J Parasitol. 1997. 35:105–110.
Article7. Katzer F, McKellar S, Kirvar E, Shiels B. Phylogenetic analysis of Theileria and Babesia equi in relation to the establishment of parasite populations within novel host species and the development of diagnostic tests. Mol Biochem Parasitol. 1998. 95:33–44.
Article8. Kawazu S, Sugimoto C, Kamio T, Fujisaki K. Analysis of the genes encoding immunodominant piroplasm surface proteins of Theileria sergenti and Theileria buffeli by nucleotide sequencing and polymerase chain reaction. Mol Biochem Parasitol. 1992. 56:169–175.
Article9. Kim GH, Lee KK, Onuma M. Susceptibility of Theileria sergenti Infection in Holstein Cattle Compared to Korean Native Cattle on Cheju Island. J Protozool Res. 1999. 9:103–112.10. Kubota S, Sugimoto C, Kakuda T, Onuma M. Analysis of immunodominant piroplasm surface antigen alleles in mixed populations of Theileria sergenti and T. buffeli. Int J Parasitol. 1996. 26:741–747.
Article11. Kubota S, Sugimoto C, Onuma M. A genetic analysis of mixed population in Theileria sergenti stocks and isolates using allele-specific polymerase chain reaction. J Vet Med Sci. 1995. 57:279–282.
Article12. Kubota S, Sugimoto C, Onuma M. Population dynamics of Theileria sergenti in persistently infected cattle and vector ticks analysed by a polymerase chain reaction. Parasitology. 1996. 112:437–442.
Article13. Matsuba T, Kubota H, Tanaka M, Hattori M, Murata M, Sugimoto C, Onuma M. Analysis of mixed parasite populations of Theileria sergenti using cDNA probes encoding a major piroplasm surface protein. Parasitology. 1993. 107:369–377.
Article14. Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988. 16:1215.
Article15. Onuma M, Kakuda T, Sugimoto C. Theileria parasite infection in East Asia and control of the disease. Comp Immunol Microbiol Infect Dis. 1998. 21:165–177.16. Rodríguez Bautista JL, Ikadai H, You M, Battsetseg B, Igarashi I, Nagasawa H, Fujisaki K. Molecular evidence of Babesia caballi (Nuttall and Strickland, 1910) parasite transmission from experimentally infected SCID mice to the ixodid tick, Haemaphysalis longicornis (Neuman, 1901). Vet Parasitol. 2001. 102:185–191.17. Sarataphan N, Kakuda T, Chansiri K, Onuma M. Survey of benign Theileria parasites of cattle and buffaloes in Thailand using allele-specific polymerase chain reaction of major piroplasm surface protein gene. J Vet Med Sci. 2003. 65:133–135.
Article18. Sarataphan N, Nilwarangkoon S, Tananyutthawongese C, Kakuda T, Onuma M, Chansiri K. Genetic diversity of major piroplasm surface protein genes and their allelic variants of Theileria parasites in Thai cattle. J Vet Med Sci. 1999. 61:991–994.
Article19. Song KH, Sang BC. Prevalence of Theileria sergenti infection in Korean native cattle by polymerase chain reaction. Korean J Parasitol. 2003. 41:141–145.
Article20. Stockham SL, Kjemtrup AM, Conrad PA, Schmidt DA, Scott MA, Robinson TW, Tyler JW, Johnson GC, Carson CA, Cuddihee P. Theileriosis in a Missouri beef herd caused by Theileria buffeli: case report, herd investigation, ultrastructure, phylogenetic analysis, and experimental transmission. Vet Pathol. 2000. 37:11–21.
Article21. Wang CT, Kubota S, Kakuda T, Kuo CC, Hsu TL, Onuma M. Survey of Theileria parasite infection in cattle in Taiwan. J Vet Med Sci. 1998. 60:253–255.22. Zhuang W, Sugimoto C, Matsuba T, Niinuma S, Murata M, Onuma M. Analyses of antigenic and genetic diversities of Theileria sergenti piroplasm surface proteins. J Vet Med Sci. 1994. 56:469–473.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The polymorphism of Theileria buffeli major surface protein associate with their clinical signs in holstein in Korea
- Molecular phylogenetic studies on clinical bovine piroplasmosis caused by benign Theileria in Shaanxi Province, China
- Expression of major piroplasm protein (p33) of Theileria sergenti (Korean isolate) and its immunogenicity in guinea pigs
- Cloning and sequencing of p33 in a Korean isolate of Theileria sergenti
- Treatment of natural tropical theileriosis with the extract of the plant Peganum harmala