J Vet Sci.
2007 Dec;8(4):409-414. 10.4142/jvs.2007.8.4.409.
The determination of dark adaptation time using electroretinography in conscious Miniature Schnauzer dogs
- Affiliations
-
- 1Department of Veterinary Surgery and Ophthalmology, College of Veterinary Medicine and BK21 Program for Veterinary Science, Seoul National University, Seoul 151-742, Korea. kmseo@snu.ac.kr
- KMID: 1106222
- DOI: http://doi.org/10.4142/jvs.2007.8.4.409
Abstract
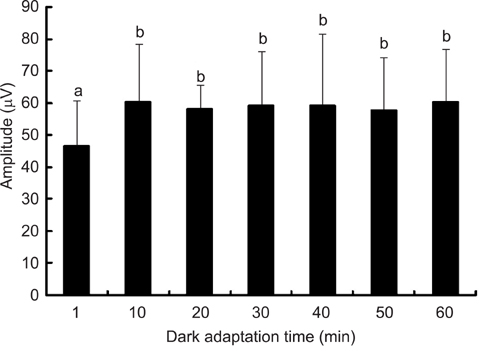
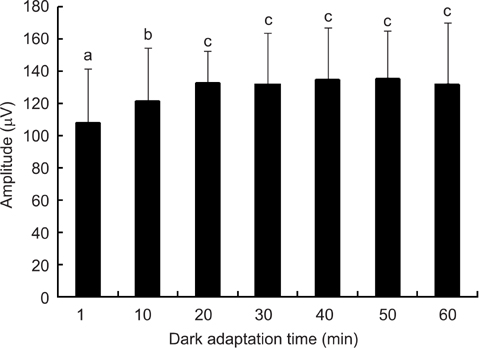
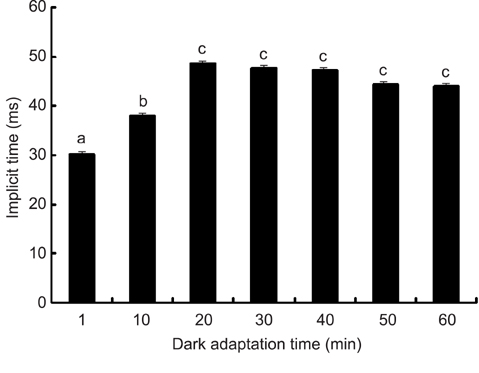
- The optimal dark adaptation time of electroretinograms (ERG's) performed on conscious dogs were determined using a commercially available ERG unit with a contact lens electrode and a built-in light source (LED-electrode). The ERG recordings were performed on nine healthy Miniature Schnauzer dogs. The bilateral ERG's at seven different dark adaptation times at an intensity of 2.5 cd.s/m2 was performed. Signal averaging (4 flashes of light stimuli) was adopted to reduce electrophysiologic noise. As the dark adaptation time increased, a significant increase in the mean a-wave amplitudes was observed in comparison to base-line levels up to 10 min (p > 0.05). Thereafter, no significant differences in amplitude occured over the dark adaptation time. Moreover, at this time the mean amplitude was 60.30 +/- 18.47 microV. However, no significant changes were observed for the implicit times of the a-wave. The implicit times and amplitude of the b-wave increased significantly up to 20 min of dark adaptation (p > 0.05). Beyond this time, the mean b-wave amplitudes was 132.92 +/- 17.79 microV. The results of the present study demonstrate that, the optimal dark adaptation time when performing ERG's, should be at least 20 min in conscious Miniature Schnauzer dogs.
MeSH Terms
Figure
Reference
-
1. Acland GM. Diagnosis and differentiation of retinal diseases in small animals by electroretinography. Semin Vet Med Surg (Small Anim). 1988. 3:15–27.2. Andréasson S, Tornqvist K, Ehinger B. Full-field electroretinograms during general anesthesia in normal children compared to examination with topical anesthesia. Acta Ophthalmol(Copenh). 1993. 71:491–495.
Article3. Dyer RS, Rigdon GC. Urethane affects the rat visual system at subanesthetic doses. Physiol Behav. 1987. 41:327–330.
Article4. Fortune B, Cull G, Wang L, Van Buskirk EM, Cioffi GA. Factors affecting the use of multifocal electroretinography to monitor function in a primate model of glaucoma. Doc Ophthalmol. 2002. 105:151–178.5. Gallemore RP, Steinberg RH. Light-evoked modulation of basolateral membrane Cl- conductance in chick retinal pigment epithelium: the light peak and fast oscillation. J Neurophysiol. 1993. 70:1669–1680.
Article6. Gum GG. Electrophysiology in veterinary ophthalmology. Vet Clin North Am Small Anim Pract. 1980. 10:437–454.
Article7. Gum GG, Gelatt KN, Samuelson DA. Maturation of the retina of the canine neonate as determined by electroretinography and histology. Am J Vet Res. 1984. 45:1166–1171.8. Hare WA, Ton H. Effects of APB, PDA, and TTX on ERG responses recorded using both multifocal and conventional methods in monkey. Effects of APB, PDA, and TTX on monkey ERG responses. Doc Ophthalmol. 2002. 105:189–222.9. Hodos W, Ghim MM, Potocki A, Fields JN, Storm T. Contrast sensitivity in pigeons: a comparison of behavioral and pattern ERG methods. Doc Ophthalmol. 2002. 104:107–118.10. Jones RD, Brenneke CJ, Hoss HE, Loney ML. An electroretinogram protocol for toxicological screening in the canine model. Toxicol Lett. 1994. 70:223–234.
Article11. Karwoski CJ, Xu X. Current source-density analysis of light-evoked field potentials in rabbit retina. Vis Neurosci. 1999. 16:369–377.
Article12. Knave B, Persson HE, Nilsson SE. The effect of barbiturate on retinal functions. II. Effects on the C-wave of the electroretinogram and the standing potential of the sheep eye. Acta Physiol Scand. 1974. 91:180–186.
Article13. Komáromy AM, Andrew SE, Sapp HL Jr, Brooks DE, Dawson WW. Flash electroretinography in standing horses using the DTL microfiber electrode. Vet Ophthalmol. 2003. 6:27–33.
Article14. Komáromy AM, Smith PJ, Brooks DE. Electroretinography in dogs and cats. Part II. Technique, interpretation, and indications. Compend Contin Educ Pract Vet. 1998. 20:355–366.15. Kommonen B. The DC-recorded dog electroretinogram in ketamine-medetomidine anaesthesia. Acta Vet Scand. 1988. 29:35–41.
Article16. Kommonen B, Karhunen U, Raitta C. Effects of thiopentone halothane-nitrous oxide anaesthesia compared to ketamine-xylazine anaesthesia on the DC recorded dog electroretinogram. Acta Vet Scand. 1988. 29:23–33.
Article17. Lam BL. Electrophysiology of Vision: Clinical Testing and Applications. 2005. Boca Raton: Taylor & Francis Group;156–159.18. Maehara S, Itoh N, Itoh Y, Wakaiki S, Tsuzuki K, Seno T, Kushiro T, Yamashita K, Izumisawa Y, Kotani T. Electroretinography using contact lens electrode with built-in light source in dogs. J Vet Med Sci. 2005. 67:509–514.
Article19. Marmor MF, Holder GE, Seeliger MW, Yamamoto S. Standard for clinical electroretinography (2004 update). Doc Ophthalmol. 2004. 108:107–114.
Article20. Narfström K, Ekesten B. Electroretinographic evaluation of Papillons with and without hereditary retinal degeneration. Am J Vet Res. 1998. 59:221–226.21. Narfström K, Ekesten B, Rosolen SG, Spiess BM, Percicot CL, Ofri R. Guidelines for clinical electroretinography in the dog. Doc Ophthalmol. 2002. 105:83–92.22. Narfström K, Nilsson SE, Andersson BE. Progressive retinal atrophy in the Abyssinian cat: studies of the DC-recorded electroretinogram and the standing potential of the eye. Br J Ophthalmol. 1985. 69:618–623.
Article23. Ofri R. Clinical electrophysiology in veterinary ophthalmology-the past, present and future. Doc Ophthalmol. 2002. 104:5–16.24. Parshall CJ, Wyman M, Nitroy S, Acland GM, Aguirre GD. Photoreceptor dysplasia: an inherited progressive retinal atrophy of Miniature Schnauzer dogs. Prog Vet Comp Ophthalmol. 1991. 1:187–203.25. Rigdon GC, Dyer RS. Ketamine alters rat flash evoked potentials. Pharmacol Biochem Behav. 1988. 30:421–426.
Article26. Rosolen SG, Rigaudiere F, Saint-Macary G, Lachapelle P. Recording the photopic electroretinogram from conscious adult Yucatan micropigs. Doc Ophthalmol. 1999. 98:197–205.27. Sasovetz D. Ketamine hydrochloride: an effective general anesthetic for use in electroretinography. Ann Ophthalmol. 1978. 10:1510–1514.28. Sato S, Sugimoto S, Chiba S. A procedure for recording electroretinogram and visual evoked potential in conscious dogs. J Pharmacol Methods. 1982. 8:173–181.
Article29. Seo KM, Kim WT, Yi NY, Jeong MB, Jeong SM, Yu HA, Nam TC. Generalized progressive retinal atrophy of dogs in Korea: 34 cases. J Vet Clin. 2004. 21:140–142.30. Sims MH. Gelatt KN, editor. Electrodiagnostic evaluation of vision. Veterinary Ophthalmology. 1999. 3rd ed. Philadelpia: Lippincott Williams & Wilkins;483–507.31. Sims MH, Brooks DE. Changes in oscillatory potentials in the canine electroretinogram during dark adaptation. Am J Vet Res. 1990. 51:1580–1586.32. Szabo-Salfay O, Palhalmi J, Szatmari E, Barabas P, Szilagyi N, Juhasz G. The electroretinogram and visual evoked potential of freely moving rats. Brain Res Bull. 2001. 56:7–14.
Article33. Textorius O, Gottvall E. The c-wave of the direct-current-recorded electroretinogram and the standing potential of the albino rabbit eye in response to repeated series of light stimuli of different intensities. Doc Ophthalmol. 1992. 80:91–103.
Article34. Ulshafer RJ, Allen C, Dawson WW, Wolf ED. Hereditary retinal degeneration in the Rhode Island Red chicken. I. Histology and ERG. Exp Eye Res. 1984. 39:125–135.
Article35. Vaegan . Electroretinograms and pattern electroretinograms of pigmented and albino rabbits. Clin Vision Sci. 1992. 7:305–311.36. Vaegan , Anderton PJ, Millar TJ. Multifocal, pattern and full field electroretinograms in cats with unilateral optic nerve section. Doc Ophthalmol. 2000. 100:207–229.37. Wongpichedchai S, Hansen RM, Koka B, Gudas VM, Fulton AB. Effects of halothane on children's electroretinograms. Ophthalmology. 1992. 99:1309–1312.
Article38. Yanase J, Ogawa H. Effects of halothane and sevoflurane on the electroretinogram of dogs. Am J Vet Res. 1997. 58:904–909.39. Yanase J, Ogawa H, Ohtsuka H. Rod and cone components in the dog electroretinogram during and after dark adaptation. J Vet Med Sci. 1995. 57:877–881.
Article40. Yanase J, Ogawa H, Ohtsuka H. Scotopic threshold response of the electroretinogram of dogs. Am J Vet Res. 1996. 57:361–366.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Radiography and ct features of atherosclerosis in two miniature schnauzer dogs
- Time of initial detection of fetal and extra-fetal structures by ultrasonographic examination in Miniature Schnauzer bitches
- Effect of the Korean Ginseng on the Normal Dark Adaptation
- Changes in ERG b-wave and Oscillatory Potential in Relation to the Dark Adaptation and Light Adaptation Time
- Specific Spectral Domain Optical Coherence Tomographic Findings of Oguchi Disease