J Vet Sci.
2010 Mar;11(1):21-25. 10.4142/jvs.2010.11.1.21.
Characterization of canine oral papillomavirus by histopathological and genetic analysis in Korea
- Affiliations
-
- 1Department of Veterinary Pathobiology, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea. jsur@konkuk.ac.kr
- 2Department of Veterinary Anatomy, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea.
- 3Department of Veterinary and Biomedical Sciences and Nebraska Center for Virology, University of Nebraska-Lincoln, Lincoln, Nebraska, 68583-0900, USA.
- KMID: 1106153
- DOI: http://doi.org/10.4142/jvs.2010.11.1.21
Abstract
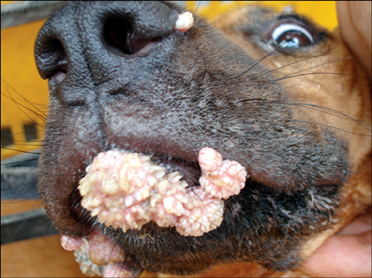
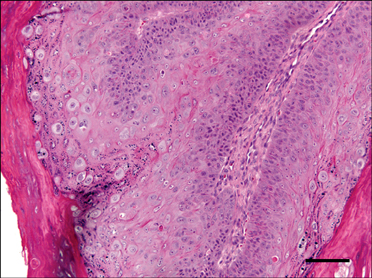
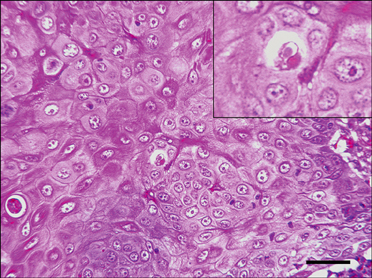
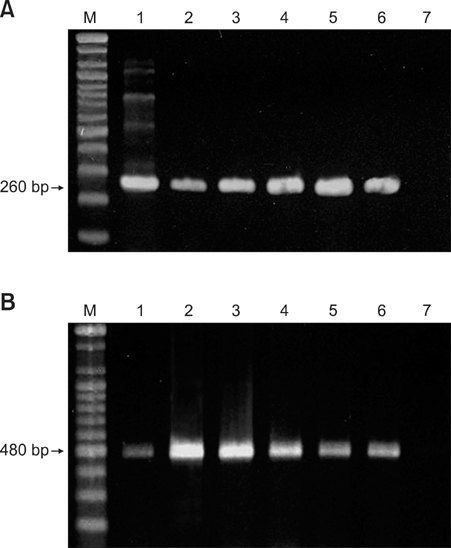
- In August 2008, forty dogs out of 400 developed oral warts in a breeding farm in Korea. Canine oral papilloma infection is a common disease in dogs. However, there has been no report of an outbreak of canine oral papillomavirus (COPV) in a group of dogs or in dog breeding farms in Korea, and the genetic analysis of COPV in Korea has yet to be performed. This study diagnosed canine oral papilloma from the oral samples of these dogs based on histopathological examination and immunohistochemistry. Polymerase chain reaction was applied to amplify the corresponding products using pre-existing primer sets for COPV and a universal human papillomavirus targeting L1 gene. Further genetic analysis of the major viral capsid gene L1 confirms the sequences of Korean COPV, which shows a close relationship to previously reported COPV. This study describes the histopathological and immunohistochemical characteristics of canine oral papilloma in a group of breeding dogs in Korea and discloses the complete L1 gene sequences of Korean COPV.
Keyword
MeSH Terms
-
Animals
Base Sequence
Capsid Proteins/chemistry/genetics
DNA, Viral/chemistry/genetics
Disease Outbreaks/*veterinary
Dog Diseases/epidemiology/*virology
Dogs
Immunohistochemistry/veterinary
Korea/epidemiology
Lambdapapillomavirus/genetics/*isolation & purification
Molecular Sequence Data
Mouth Diseases/epidemiology/*veterinary/virology
Papillomavirus Infections/epidemiology/*veterinary/virology
Polymerase Chain Reaction/veterinary
Sequence Analysis, DNA
Figure
Reference
-
1. Campbell KL, Sundberg JP, Goldschmidt MH, Knupp C, Reichmann ME. Cutaneous inverted papillomas in dogs. Vet Pathol. 1988. 25:67–71.
Article2. de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004. 324:17–27.
Article3. Delius H, Van Ranst MA, Jenson AB, zur Hausen H, Sundberg JP. Canine oral papillomavirus genomic sequence: a unique 1.5-kb intervening sequence between the E2 and L2 open reading frames. Virology. 1994. 204:447–452.
Article4. Forslund O, Antonsson A, Nordin P, Stenquist B, Hansson BG. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J Gen Virol. 1999. 80:2437–2443.
Article5. Goldstein DJ, Finbow ME, Andresson T, McLean P, Smith K, Bubb V, Schlegel R. Bovine papillomavirus E5 oncoprotein binds to the 16K component of vacuolar H+-ATPases. Nature. 1991. 352:347–349.
Article6. Head KW, Else RW, Dubielzig RR. Meuten DJ, editor. Tumors of the alimentary tract. Tumors in Domestic Animals. 2002. 4th ed. Ames: Iowa State University Press;422–423.
Article7. Isegawa N, Nakano K, Ohta M, Shirasawa H, Tokita H, Simizu B. Cloning and sequencing of the L1 gene of canine oral papillomavirus. Gene. 1994. 146:261–265.
Article8. Masterson PJ, Stanley MA, Lewis AP, Romanos MA. AC-terminal helicase domain of the human papillomavirus E1 protein binds E2 and the DNA polymerase alpha-primase p68 subunit. J Virol. 1998. 72:7407–7419.
Article9. Narama I, Kobayashi Y, Yamagami T, Ozaki K, Ueda Y. Pigmented cutaneous papillomatosis (pigmented epidermal nevus) in three pug dogs; histopathology, electron microscopy and analysis of viral DNA by the polymerase chain reaction. J Comp Pathol. 2005. 132:132–138.
Article10. Nicholls PK, Klaunberg BA, Moore RA, Santos EB, Parry NR, Gough GW, Stanley MA. Naturally occurring, nonregressing canine oral papillomavirus infection: host immunity, virus characterization, and experimental infection. Virology. 1999. 265:365–374.
Article11. Nicholls PK, Stanley MA. Canine papillomavirus - A centenary review. J Comp Pathol. 1999. 120:219–233.12. Shimada A, Shinya K, Awakura T, Narama I, Maeda H, Umemura T. Cutaneous papillomatosis associated with papillomavirus infection in a dog. J Comp Pathol. 1993. 108:103–107.
Article13. Tanabe C, Kano R, Nagata M, Nakamura Y, Watanabe S, Hasegawa A. Molecular characteristics of cutaneous papillomavirus from the canine pigmented epidermal nevus. J Vet Med Sci. 2000. 62:1189–1192.
Article14. Teifke JP, Löhr CV, Shirasawa H. Detection of canine oral papillomavirus-DNA in canine oral squamous cell carcinomas and p53 overexpressing skin papillomas of the dog using the polymerase chain reaction and non-radioactive in situ hybridization. Vet Microbiol. 1998. 60:119–130.
Article15. Yuan H, Ghim S, Newsome J, Apolinario T, Olcese V, Martin M, Delius H, Felsburg P, Jenson B, Schlegel R. An epidermotropic canine papillomavirus with malignant potential contains an E5 gene and establishes a unique genus. Virology. 2007. 359:28–36.
Article16. Zhou J, Sun XY, Louis K, Frazer IH. Interaction of human papillomavirus (HPV) type 16 capsid proteins with HPV DNA requires an intact L2 N-terminal sequence. J Virol. 1994. 68:619–625.
Article17. zur Hausen H. Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Curr Top Microbiol Immunol. 1994. 186:131–156.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Why Should You Care About Oral Gonorrhea and Oral Human Papillomavirus Infection?
- Recharacterization of the Canine Adenovirus Type 1 Vaccine Strain based on the Biological and Molecular Properties
- Management of Maxillary Impacted Canines
- ERRATUM: Isolation and characterization of canine umbilical cord blood-derived mesenchymal stem cells
- New genotype classification and molecular characterization of canine and feline parvoviruses