J Vet Sci.
2007 Mar;8(1):7-14. 10.4142/jvs.2007.8.1.7.
Comparative analysis of heart functions in micropigs and conventional pigs using echocardiography and radiography
- Affiliations
-
- 1College of Veterinary Medicine, Biotherapy Human Resources Center (BK21), Chonnam National University, Gwangju 500-757, Korea. hjhan@chonnam.ac.kr
- 2Department of Cardiology, Chonnam National University Hospital, Chonnam National University, Gwangju 500-757, Korea.
- KMID: 1104230
- DOI: http://doi.org/10.4142/jvs.2007.8.1.7
Abstract
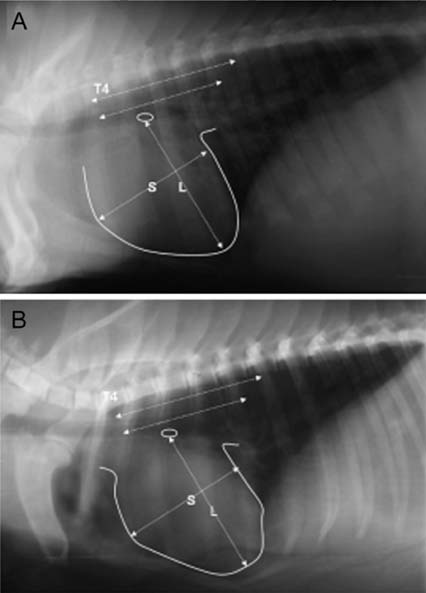
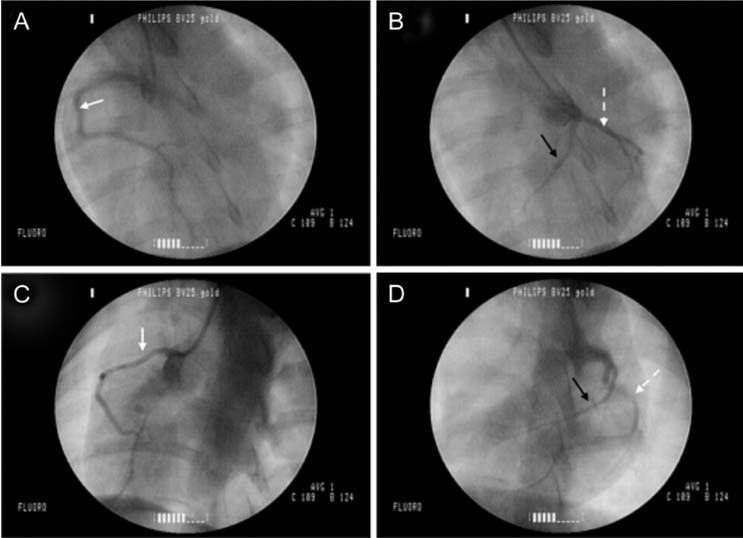
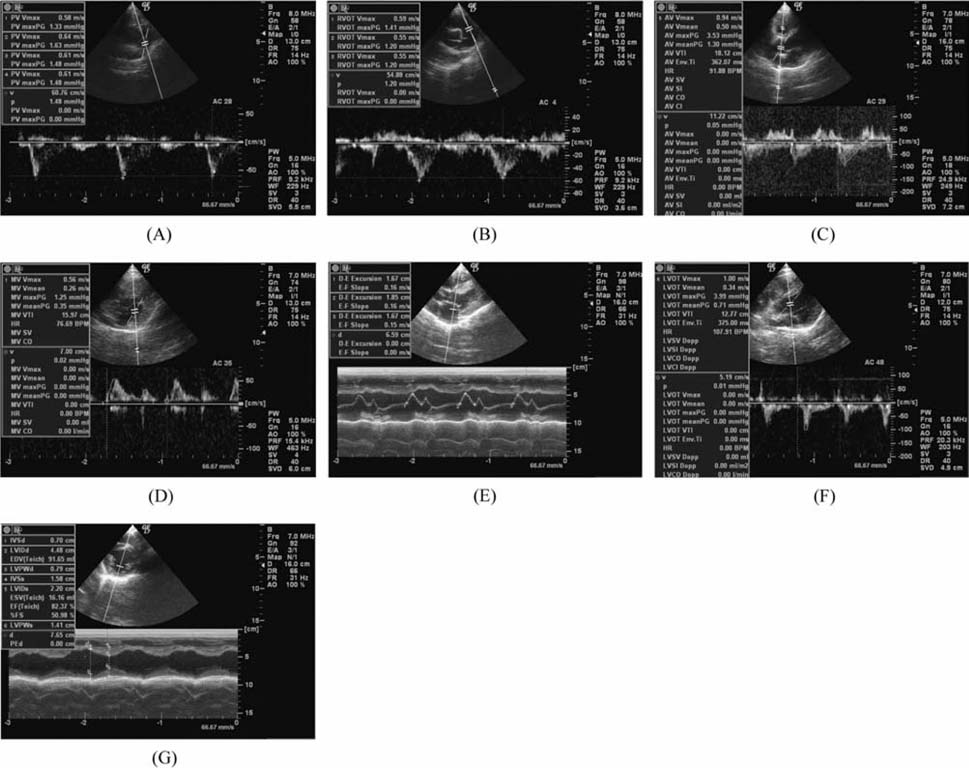
- The production of miniature animals has been suggested for use in organ transplantation. At present, many of the studies about application of animal organs to human have been focused on pigs because of the number of advantages involved and due to their similarities with human. However, a physiological analysis of the organs to be transplanted has not yet been carried out. Therefore, this study analyzed whether or not there were physiological and morphological differences in the hearts of conventionallyreared pigs and micropigs. In this study, the morphological and physiological functions of the heart were examined using radiographic and echocardiographic equipment. In the lateral radiographic view, the heart of the micropig has a larger cardiac long axis : short axis ratio than does the conventional pig, but the difference in the vertebral heart score was not significant. In addition, there were no morphological differences on the X-ray fluoroscopic view. There were no differences in echocardiographic values, except for several values in the left ventricle traces. Overall, it is expected that the values measured in this study will contribute to understanding of the physiological characteristics of micropigs.
MeSH Terms
Figure
Reference
-
1. Benzing G 3rd, Stockert J, Nave E, Tsuei YG, Kaplan S. Evaluation of canine left ventricular contractility. Cardiovasc Res. 1974. 8:313–322.
Article2. Bhatti FN, Schmoeckel M, Zaidi A, Cozzi E, Chavez G, Goddard M, Dunning JJ, Wallwork J, White DJ. Three-month survival of HDAFF transgenic pig hearts transplanted into primates. Transplant Proc. 1999. 31:958.
Article3. Buchanan JW, Bucheler J. Vertebral scale system to measure canine heart size in radiographs. J Am Vet Med Assoc. 1995. 206:194–199.4. Crick SJ, Sheppard MN, Ho SY, Gebstein L, Anderson RH. Anatomy of the pig heart: Comparisons with normal human cardiac structure. J Anat. 1998. 193:105–119.
Article5. Ettinger SJ. Textbook of Veterinary Internal Medicine. 1989. 3rd ed. Philadelphia: Saunders;923–938.6. Evans RW, Orians CE, Ascher NL. The potential supply of organ donors. An assessment of the efficacy of organ procurement efforts in the United States. JAMA. 1992. 267:239–246.
Article7. Gwathmey JK, Nakao S, Come PC. Echocardiographic assessment of cardiac chamber size and functional performance in swine. Am J Vet Res. 1989. 50:192–197.8. Hardy MA. Xenograft 25. 1989. Amsterdam: Elsevier;125.9. Korte FS, Mokelke EA, Sturek M, McDonald KS. Exercise improves impaired ventricular function and alterations of cardiac myofibrillar proteins in diabetic dyslipidemic pigs. J Appl Physiol. 2005. 98:461–467.
Article10. Kaczmarek I, Feindt P, Boeken U, Guerler S, Gams E. Effects of direct mechanical ventricular assistance on regional myocardial function in an animal model of acute heart failure. Artif Organs. 2003. 27:261–266.
Article11. Lessick J, Hayam G, Zaretsky A, Reisner SA, Schwartz Y, Ben-Heim SA. Evaluation of inotropic changes in ventricular function by NOGA maping: comparison with echocardiography. J Appl Physiol. 2002. 93:418–426.
Article12. Lundeen G, Manohar M, Parks C. Systemic distribution of blood flow in swine while awake or during 1.0 and 1.5 MAC isoflurane anesthesia with or without 50% nitrous oxide. Anesth Analg. 1983. 62:499–512.13. Park SH, Kim DY, Park BK, Yoo HS, Youn HJ, Han HJ. Comparative analysis of various blood chemical values between Miniature pigs and conventional pigs. Lab Anim Res. 2006. 22:19–23.14. Park SW. Multicenter trial for estimation of normal values of echocardiographic indices in Korea. Korean Circ J. 2000. 30:373–382.
Article15. Pipers FS, Muir WW, Hamlin RL. Echocardiography in swine. Am J Vet Res. 1978. 39:707–710.16. Snedecor GW, Cochran WG. Statistical Methods. 1967. 7th ed. Edinburgh: Oliver & Boyd;156–236.17. Stankovicova T, Szilard M, De Scheeder I, Sipido KR. M cells and transmural heterogeneity of action potential configuration in myocytes from the left ventricular wall of the pig heart. Cardiovasc Res. 2000. 45:952–960.
Article18. Strotman JM, Janerot-Sjoberg B, Kimme P, Frohlich B, Voigt JU, Schreckenberger AB, Hatle L, Sutherland GR. The effet of pacing-induced heart rate variation on longitudinal and circumferential regional myocardial function after acute beta-blockade-acardiac ultrasound study. Eur J Echocardiogr. 2000. 1:184–195.19. Vogel M, Schmidt MR, Kristiansen SB, Cheung M, White PA, Sorensen K, Redington AN. Validation of myocardial acceleration during isovolumic contraction as a novel noninvasive index of right ventricular contractility: comparison with ventricular pressure-volume relations in an animal model. Circulation. 2002. 105:1693–1699.
Article20. Weidemann F, Jamal F, Sutherland GR, Claus P, Kowalski M, Hatle L, De Scheerder I, Bijnens B, Rademakers FE. Myocardial function defined by strain rate and strain during alterations in inotropic states and heart rate. Am J Physiol Heart Circ Physiol. 2002. 283:H792–H799.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of cardiac function and coronary angiography between conventional pigs and micropigs as measured by multidetector row computed tomography
- Effectiveness of 99mTc-tetrofosmin for assessment of heart functions in micropigs
- Echocardiographic Evaluation of the Right Heart
- Recent Advances in Echocardiography
- Echocardiographic Findings of Heart Disease in Children