Korean J Ophthalmol.
2005 Mar;19(1):9-17. 10.3341/kjo.2005.19.1.9.
Fibrovascularization of Intraorbital Hydroxyapatite-Coated Alumina Sphere in Rabbits
- Affiliations
-
- 1Dr. Chung's Eye Clinic, Taegu, Korea. eye-chung@hanmail.net
- 2Department of Ophthalmology, College of Medicine, Seoul National University, Seoul, Korea.
- 3Department of Ophthalmology, College of Medicine, Yeungnam University, Taegu, Korea.
- KMID: 1099079
- DOI: http://doi.org/10.3341/kjo.2005.19.1.9
Abstract
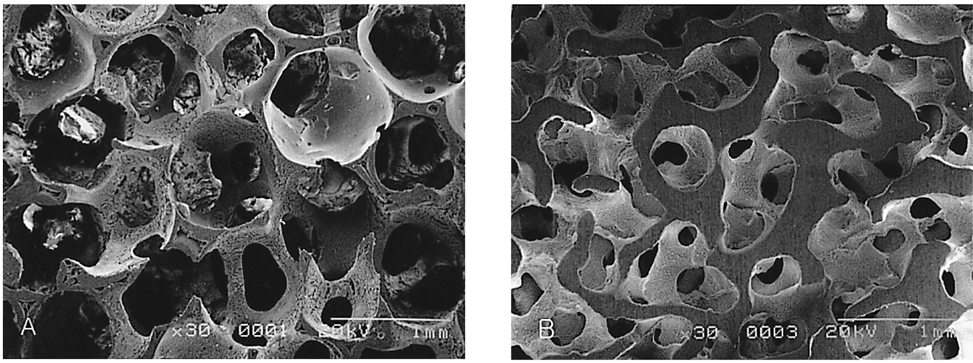
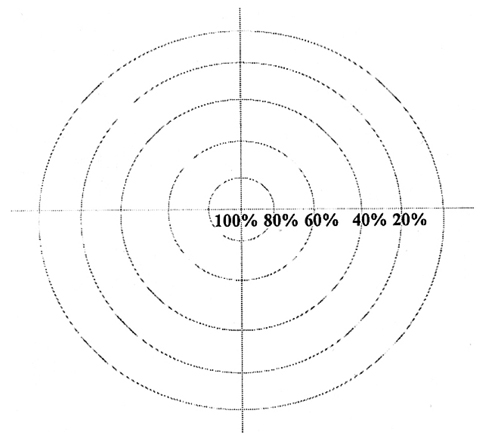
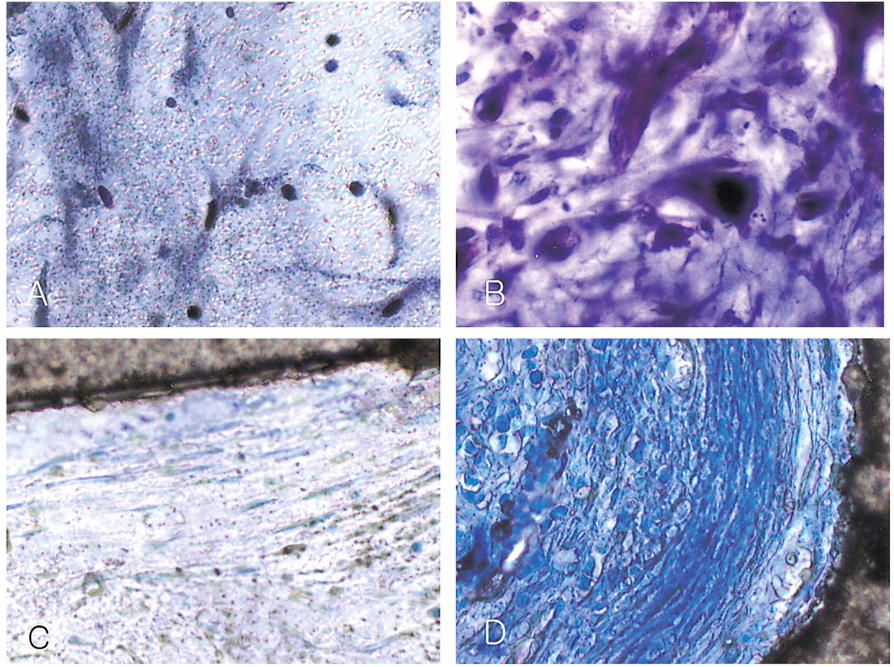
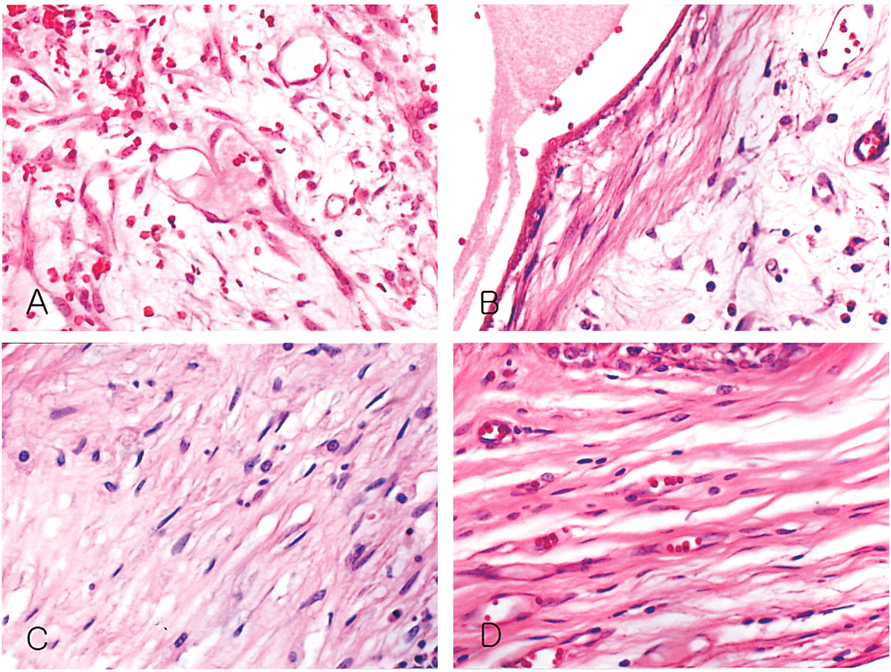
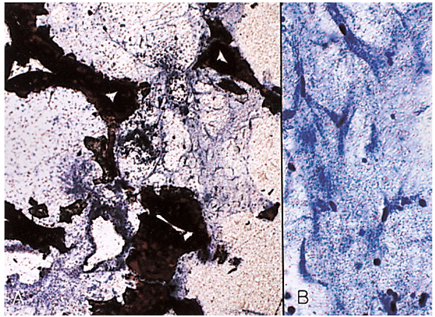
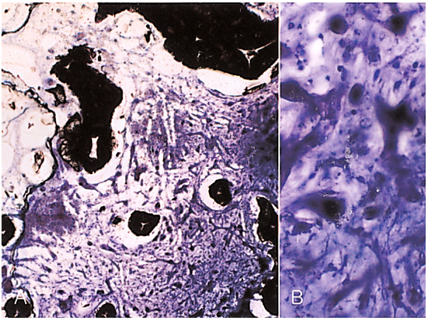
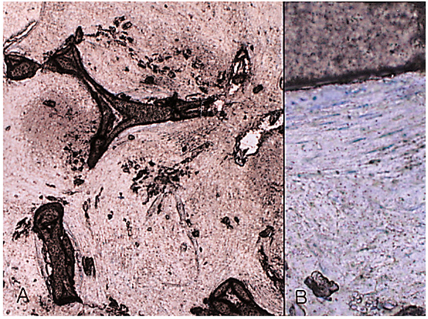
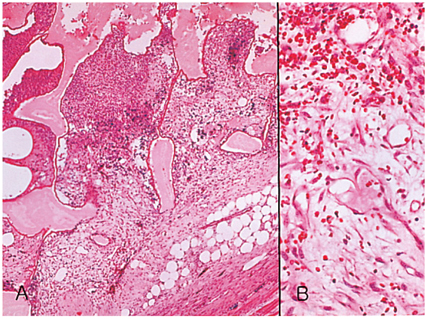
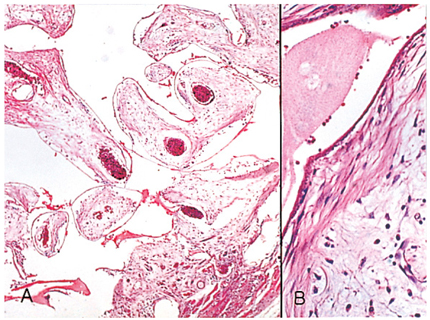
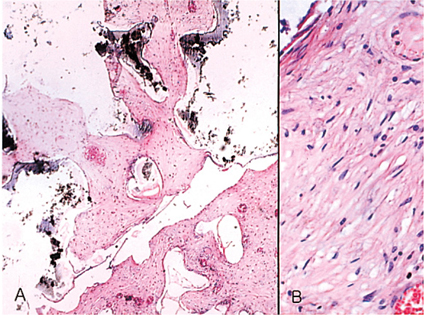
- We investigated the fibrovascular ingrowth and fibrovascular tissue maturation of hydroxyapatite-coated, porous alumina sphere (Alumina sphere) in comparison with the hydroxyapatite sphere (HAp sphere) in rabbits. Alumina spheres and HAp spheres were implanted in the left orbits of 42 New Zealand white rabbits after enucleation. Fibrovascular ingrowth and maturation were graded from 1 to 5 at postoperative 1, 2, 3, 4, 8, 12 and 24 weeks. We defined 4 phases: postoperative 1-2 weeks as phase I, 3-4 weeks as phase II, 8-12 weeks as phase III, and 24 weeks as phase IV. The grade was analyzed at each phases. There was no significant difference in fibrovascular ingrowth and maturation between the two groups at all 4 phases, except phase II at which the Alumina sphere showed significantly lower maturation grade (p< 0.05). We concluded that the Alumina sphere is an ideal orbital implant material and an ideal substitute for the HAp sphere in clinical practice.
Keyword
MeSH Terms
Figure
Reference
-
1. Durham DG. The new ocular implants. Am J Ophthalmol. 1949. 32:79–89.2. Shields CL, Shields JA, De Potter P. Hydroxyapatite orbital implant after enucleation: experience with initial 100 consecutive cases. Arch Ophthalmol. 1992. 110:333–338.3. Rubin PA, Nicaeus TE, Warner MA, Remulla HO. Effect of sucralfate and basic fibroblast growth factor on fibrovascular ingrowth into hydroxyapatite and porous polyethylene alloplastic implants using novel rabbit model. Ophthal Plast Reconstr Surg. 1997. 13:8–17.4. Byrd WA. Coraline hydroxyapatite orbital implant. Ophthal Practice. 1991. 9:262–266.5. Cutton JJ. Coraline hydroxyapatite as an ocular implant. Ophthalmology. 1991. 98:370–377.6. Perry AR. Advances in enucleation. Ophthal Plast Reconstr Surg. 1991. 4:173–182.7. Roy DM, Linnehan SK. Hydroxyapatite formed from coral skeletal carbonate by hydrothermal exchange. Nature. 1974. 247:220–222.8. You CK, Oh SH, Kim SY. Hydroxyapatite coated porous alumina as a new orbital implant. Key Engineering Materials. 2003. 240–242. 563–566.9. Hornblass A, Biesman B, Eviatar J. Current techniques of enucleation: a summary of 5439 intraorbital implants and a review of the literature. Ophthal Plast Reconstr Surg. 1995. 11:77–88.10. Fricain J, Baquei C, Dupuy B. Resorption of corals implanted in diffusion chambers. J Mater Sci Mater Med. 1995. 6:680–684.11. Mawn LA, Jordan DR, Gilberg S. Scanning electron microscopic examination of porous orbital implants. Can J Ophthalmol. 1998. 33:203–209.12. Jordan DR, Mawn LA, Brownstein S, et al. The bioceramic orbital implant: a new generation of porous implants. Ophthal Plast Reconstr Surg. 2000. 16:347–355.13. Jordan DR, Gilberg S, Mawn LA. The bioceramic orbital implant. Experience with 107 implants. Ophthal Plast Reconstr Surg. 2003. 19:128–135.14. Bose MO, Avers RJ, Rieger MR, Duckworth JE. Submerged alumina dental root implants in humans: five-year evaluation. J Prosthet Dent. 1989. 61:594–601.15. Sclatter C. Biomedical aspects of aluminum. Med Lav. 1992. 83:470–474.16. Heimke G, Griss P, Jentschura G, Werner E. Bioinert and bioactive ceramics in orthopaedic surgery. Mechanical properties of biomaterials. 1980. Toronto: John Willey & Sons;207–215.17. Labat B, Chamson A, Frey J. Effects of γ-alumina and hydroxyapatite coatings on the growth and metabolism of human osteoblasts. J Biomed Mater Res. 1995. 29:1397–1401.18. Rubin PA, Popham JK, Bilyk JR, Shore JW. Comparison of fibrovascular ingrowth into hydroxyapatite and porous polyethylene orbital implants. Ophthal Plast Reconstr Surg. 1994. 10:96–103.19. Rubin PA, Bilyk JR, Shore JW. Orbital reconstruction using porous polyethylene sheets. Ophthalmology. 1994. 101:1697–1708.20. Seong YS, Lee SY, Kim SJ. Morphological study of a new orbital implant: hydroxyapatite-coated porous alumina in rabbit. J Korean Ophthalmol Soc. 2001. 42:1354–1361.21. Jordan DR, Brownstein S, Gilberg S, et al. Hydroxyapatite and calcium phosphate coatings on aluminium oxide orbital implants. Can J Ophthalmol. 2001. 37:7–13.22. Mawn LA, Jordan DR, Gilberg S. Scanning electron microscopic examination of porous orbital implants. Can J Ophthalmol. 1998. 33:203–209.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Morphological Study of a New Orbital Implant: Hydroxyapatite-Coated Porous Alumina in Rabbit
- Fibrovascular Ingrowth of the Orbital Hydroxyapatite Implant evaluated by Magnetic Resonance Imaging
- MRI Findings of Orbital Hydroxyapatite Implants in Postoperative 5 months
- Effect of Collagen Gel with Growth Factor and Wrapping Materials on Fibrovascularization of Porous Orbital Implant
- Histopathologic Comparison of Vascularization between Dacron and Donor Sclera as Wrapping Material in Hydroxyapatite Implantation