Korean J Radiol.
2008 Apr;9(2):162-174. 10.3348/kjr.2008.9.2.162.
Intrapancreatic Accessory Spleen: Findings on MR Imaging, CT, US and Scintigraphy, and the Pathologic Analysis
- Affiliations
-
- 1Department of Radiology, Seoul National University Hospital, Seoul, Korea. leejm@radcom.snu.ac.kr
- 2Institute of Radiation Medicine, Seoul National University Hospital, Seoul, Korea.
- 3Department of Radiology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 4Department of Radiology, Section of Digestive Diseases, University Hospital, UMD-New Jersey Medical School, Newark, New Jersey, USA.
- KMID: 1098196
- DOI: http://doi.org/10.3348/kjr.2008.9.2.162
Abstract
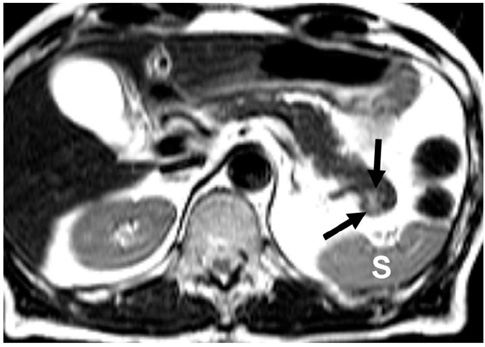
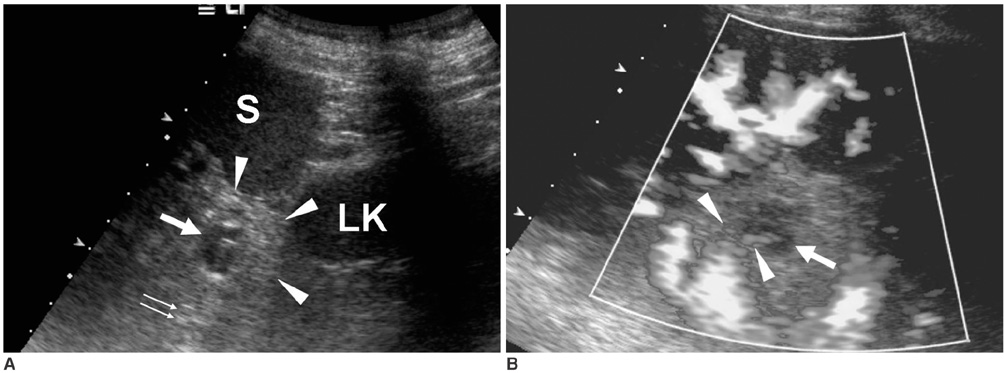
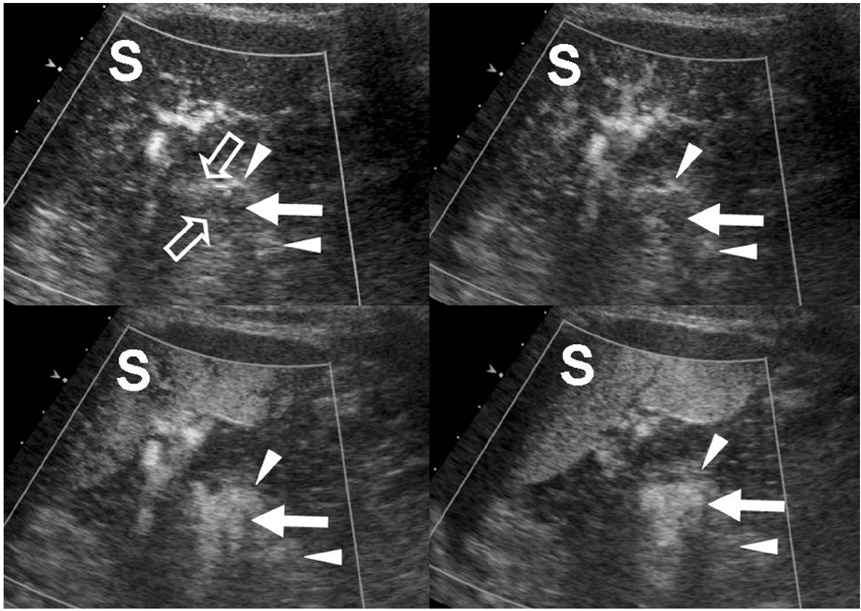
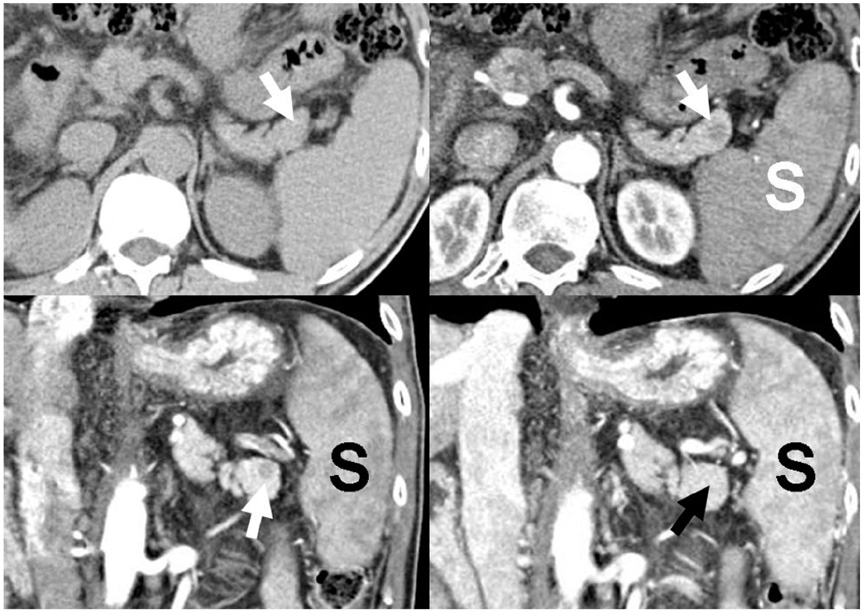
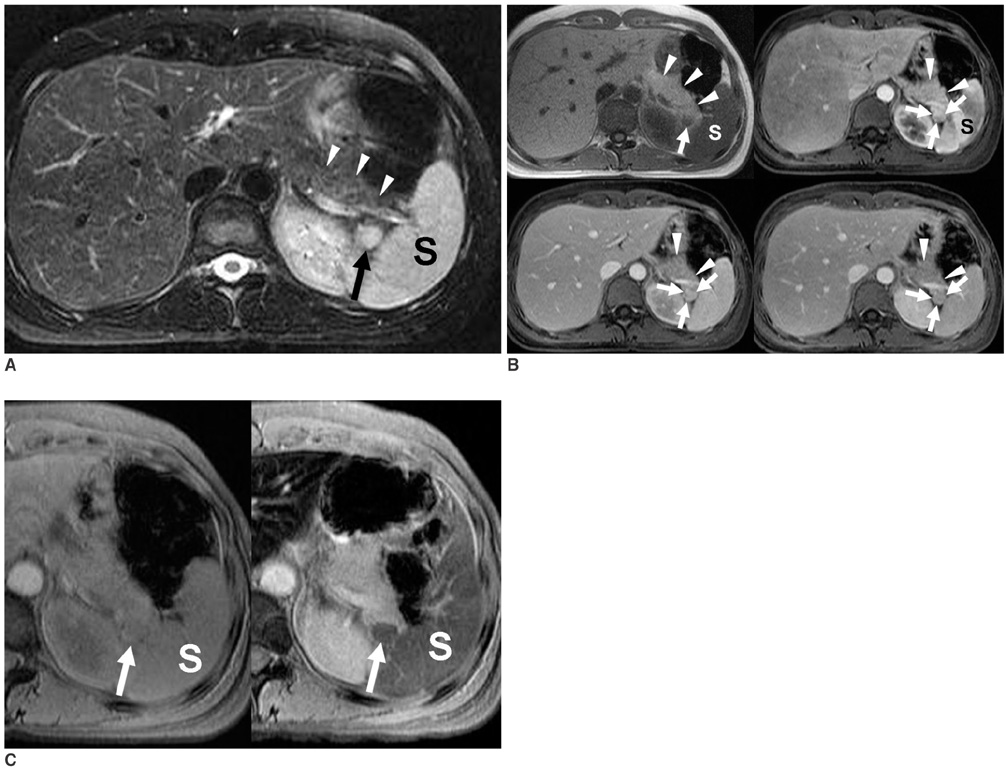
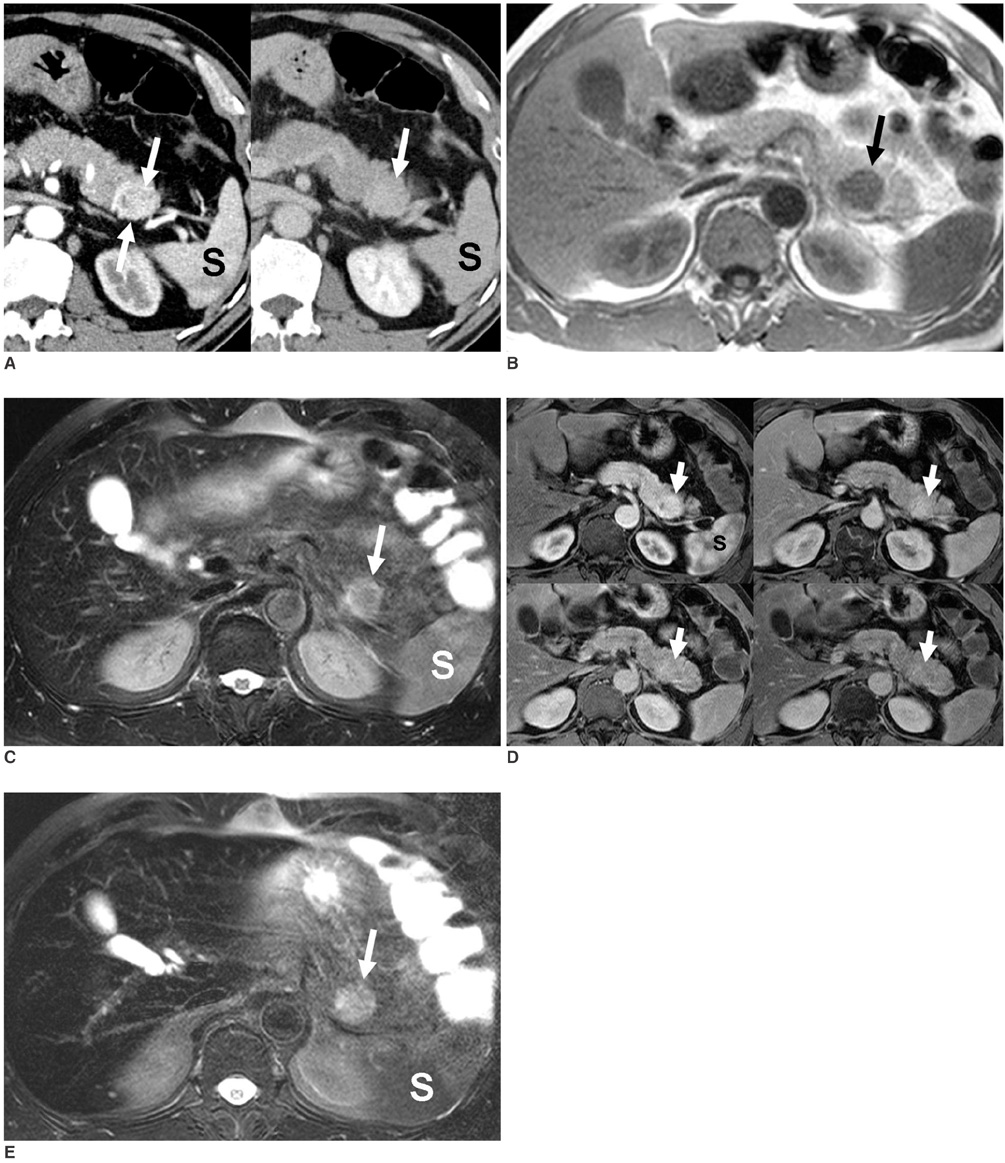
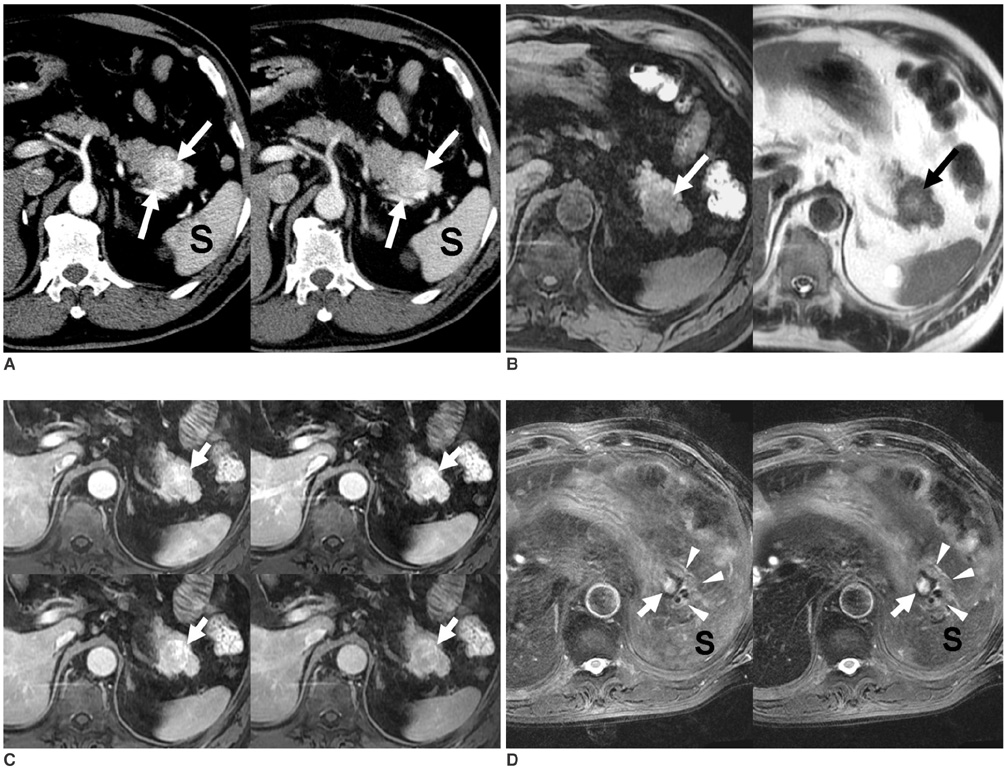
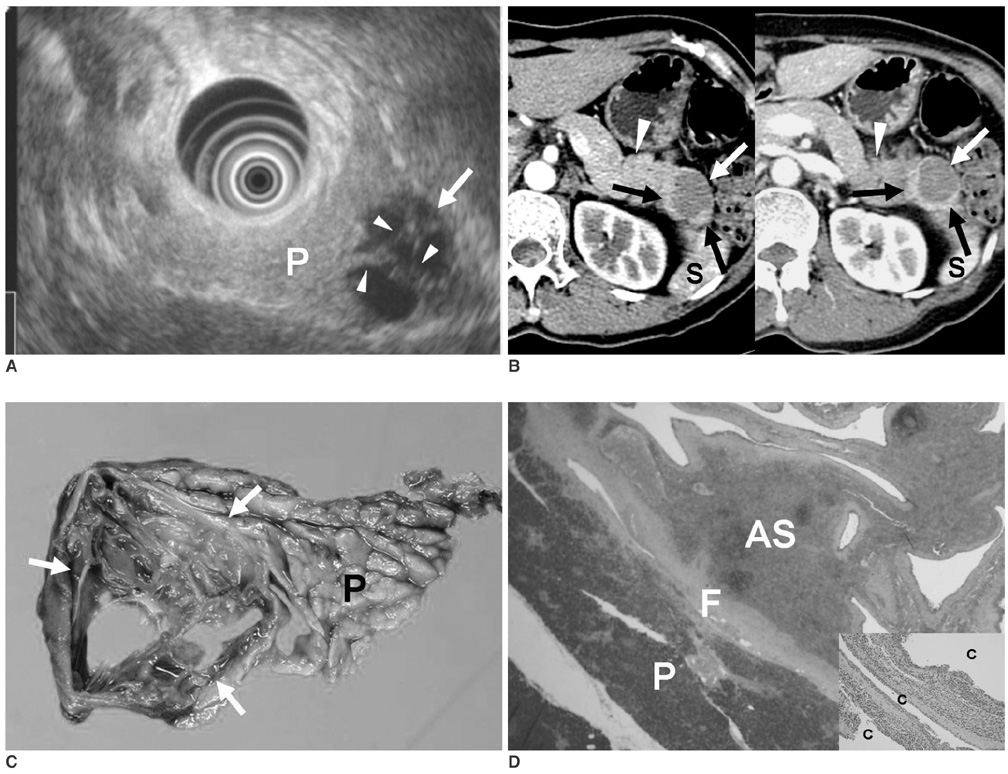
- Although the tail of the pancreas is the second most common site of an accessory spleen, intrapancreatic accessory spleen (IPAS) has rarely been noted radiologically. However, as the imaging techniques have recently advanced, IPAS will be more frequently detected as an incidental pancreatic nodule on CT or MRI. Because accessory spleens usually pose no clinical problems, it is important to characterize accessory spleens as noninvasively as possible. An IPAS has similar characteristics to those of the spleen on the precontrast and contrast-enhanced images of all the imaging modalities. In particular, inhomogeneous enhancement of an IPAS in its early phases may be a diagnostic clue. Superparamagnetic iron oxide (SPIO)-enhanced MRI and Levovist-enhanced US, and the mechanisms of which are theoretically similar to that of Tc-99m scintigraphy, can be used as alternative tools to confirm the diagnosis of IPAS. An IPAS shows a significant signal drop similar to the spleen on the SPIO-enhanced T2 or T2*-weighted imaging and prolonged enhancement on the delayed hepatosplenic phase of contrast-enhanced US. We review and illustrate the differential points between IPAS and hypervascular pancreatic tumors in this manuscript.
MeSH Terms
Figure
Cited by 3 articles
-
Epidermoid Cyst Arising from Intrapancreatic Accessory Spleen
Jin-A Ryoo, Seung Soo Kim
Korean J Gastroenterol. 2020;76(5):265-267. doi: 10.4166/kjg.2020.131.A Case of Intrapancreatic Accessory Spleen Mistaken as a Pancreatic Mass due to Different Enhancing Pattern from Normal Spleen
Jun Seok Park, Wan Jung Kim, Yeong Gyu Jeong, Youn Sun Park, Hyun Cheol Koo, Tae Il Lee, Gyo Chang Choi, Sook Kim
Korean J Gastroenterol. 2011;58(6):357-360. doi: 10.4166/kjg.2011.58.6.357.성인에서 부비장 경색으로 인해 발생한 급성 복통 1예
Hoyoung Wang, Hoonsub So, Yang Won Nah, Misung Kim, Tae Young Lee, Minjung Seo, Sung-Jo Bang
Korean J Gastroenterol. 2021;78(3):183-187. doi: 10.4166/kjg.2021.071.
Reference
-
1. Movitz D. Accessory spleens and experimental splenosis. Principles of growth. Chic Med Sch Q. 1967. 26:183–187.2. Halpert B, Gyorkey F. Lesions observed in accessory spleens of 311 patients. Am J Clin Pathol. 1959. 32:165–168.3. Eraklis AJ, Filler RM. Splenectomy in childhood: a review of 1413 cases. J Pediatr Surg. 1972. 7:382–388.4. Harris GN, Kase DJ, Bradnock H, Mckinley MJ. Accessory spleen causing a mass in the tail of the pancreas: MR imaging findings. AJR Am J Roentgenol. 1994. 163:1120–1121.5. Hamada T, Isaji S, Mizuno S, Tabata M, Yamagiwa K, Yokoi H, et al. Laparoscopic spleen-preserving pancreatic tail resection for an intrapancreatic accessory spleen mimicking a nonfunctioning endocrine tumor: report of a case. Surg Today. 2004. 34:878–881.6. Davidson ED, Campbell WG, Hersh T. Epidermoid splenic cyst occurring in an intrapancreatic accessory spleen. Dig Dis Sci. 1980. 25:964–967.7. Kim SH, Lee JM, Han JK, Lee JY, Kang WJ, Jang JY, et al. MDCT and superparamagnetic iron oxide (SPIO)-enhanced MR findings of intrapancreatic accessory spleen in seven patients. Eur Radiol. 2006. 16:1887–1897.8. Subramanyam BR, Balthazar EJ, Horii SC. Sonography of the accessory spleen. AJR Am J Roentgenol. 1984. 143:47–49.9. Kim SH, Lee JM, Lee JY, Han JK, Choi BI. Contrast-enhanced sonography of intrapancreatic accessory spleen in six patients. AJR Am J Roentgenol. 2007. 188:422–428.10. Paterson A, Frush DP, Donnelly LF, Foss JN, O'Hara SM, Bisset GS 3rd. A pattern-oriented approach to splenic imaging in infants and children. Radiographics. 1999. 19:1465–1485.11. Blomley MJ, Kormano M, Coulden R, Lim-Dunham J, Dawson P, Lipton MJ. Splenic blood flow: evaluation with computed tomography. Acad Radiol. 1997. 4:13–20.12. Stabile Ianora AA, Muscogiuri E, Scardapane A, Angelelli G. Multislice CT in the study of insulinomas: preliminary experience. Radiol Med (Torino). 2004. 107:325–331.13. Ichikawa T, Peterson MS, Federle MP, Baron RL, Haradome H, Kawamori Y, et al. Islet cell tumor of the pancreas: biphasic CT versus MR imaging in tumor detection. Radiology. 2000. 216:163–171.14. Sica GT, Reed MF. Case 27: intrapancreatic accessory spleen. Radiology. 2000. 217:134–137.15. Ota T, Tei M, Yoshioka A, Mizuno M, Watanabe S, Seki M, et al. Intrapancreatic accessory spleen diagnosed by technetium-99m heat-damaged red blood cell SPECT. J Nucl Med. 1997. 38:494–495.16. Ding H, Kudo M, Onda H, Nomura H, Haji S. Sonographic diagnosis of pancreatic islet cell tumor: value of intermittent harmonic imaging. J Clin Ultrasound. 2001. 29:411–416.17. Van Hoe L, Gryspeerdt S, Marchal G, Baert AL, Mertens L. Helical CT for the preoperative localization of islet cell tumors of the pancreas: value of arterial and parenchymal phase images. AJR Am J Roentgenol. 1995. 165:1437–1439.18. Keogan MT, McDermott VG, Paulson EK, Sheafor DH, Frederick MG, de Long DM, et al. Pancreatic malignancy: effect of dual-phase helical CT in tumor detection and vascular opacification. Radiology. 1997. 205:513–518.19. Carlson B, Johnson CD, Stephens DH, Ward EM, Kvols LK. MRI of pancreatic islet cell carcinoma. J Comput Assist Tomogr. 1993. 17:735–740.20. Sahani D, Prasad SR, Maher M, Warshaw AL, Hahn PF, Saini S. Functioning acinar cell pancreatic carcinoma: diagnosis on mangafodipir trisodium (Mn-DPDP)-enhanced MRI. J Comput Assist Tomogr. 2002. 26:126–128.21. Ng CS, Loyer EM, Iyer RB, David CL, DuBrow RA, Charnsangavej C. Metastases to the pancreas from renal cell carcinoma: findings on three-phase contrast-enhanced helical CT. AJR Am J Roentgenol. 1999. 172:1555–1559.22. Kelekis NL, Semelka RC, Siegelman ES. MRI of pancreatic metastases from renal cancer. J Comput Assist Tomogr. 1996. 20:249–253.23. Higaki K, Jimi A, Watanabe J, Kusaba A, Kojiro M. Epidermoid cyst of the spleen with CA19-9 or carcinoembryonic antigen productions: report of three cases. Am J Surg Pathol. 1998. 22:704–708.24. Sonomura T, Kataoka S, Chikugo T, Hirooka T, Makimoto S, Nakamoto T, et al. Epidermoid cyst originating from an intrapancreatic accessory spleen. Abdom Imaging. 2002. 27:560–562.25. Horibe Y, Murakami M, Yamao K, Imaeda Y, Tashiro K, Kasahara M. Epithelial inclusion cyst (epidermoid cyst) formation with epithelioid cell granuloma in an intrapancreatic accessory spleen. Pathol Int. 2001. 51:50–54.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Intrapancreatic Accessory Spleen Mistaken as a Pancreatic Mass due to Different Enhancing Pattern from Normal Spleen
- Malignant Transformation of an Epidermoid Cyst in an Intrapancreatic Accessory Spleen: A Case Report
- Epithelial Cysts in the Intrapancreatic Accessory Spleen that Clinically Mimic Pancreatic Cystic Tumor: A Report of Two Cases
- Two Cases of Epidermoid Cysts in the Intrapancreatic Accessory Spleen Mimicking Pancreatic Cystic Neoplasm
- Spleen Scan for 68Ga-DOTATOC PET-Positive Pancreatic Tail Lesion: Differential Diagnosis of Neuroendocrine Tumor from Accessory Spleen