Korean J Lab Med.
2010 Apr;30(2):171-177. 10.3343/kjlm.2010.30.2.171.
Clinical Usefulness of T-SPOT.TB Test for the Diagnosis of Tuberculosis
- Affiliations
-
- 1Department of Laboratory Medicine, Hallym University College of Medicine, Seoul, Korea. hskim0901@empal.com
- 2Department of Internal Medicine, Hallym University College of Medicine, Seoul, Korea. kimch2002@hallym.or.kr
- 3Department of Laboratory Medicine, Konkuk University School of Medicine, Seoul, Korea.
- KMID: 1096804
- DOI: http://doi.org/10.3343/kjlm.2010.30.2.171
Abstract
- BACKGROUND
Tuberculosis-specific ELISPOT assay (T-SPOT.TB, Oxford Immunotec, UK) is a test that detects interferon-gamma producing T-cells after stimulating patient's lymphocytes with two kinds of tuberculosis-specific antigens (ESAT-6 and CFP-10). We evaluated clinical usefulness of T-SPOT.TB test in Korean adults with intermediate burden of tuberculosis and high rate of BCG vaccination at birth.
METHODS
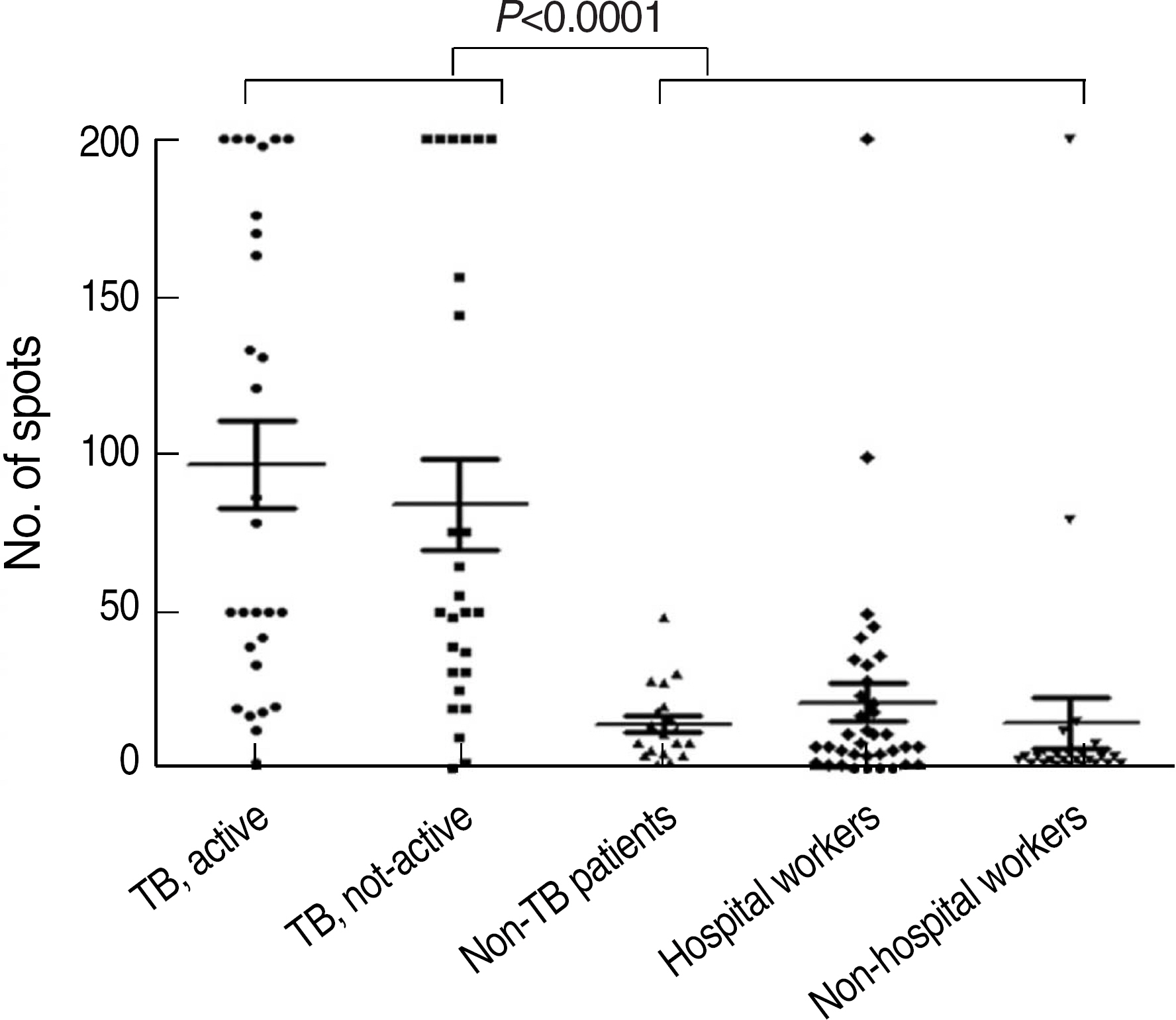
T-SPOT.TB test was performed in 79 patients and 64 healthy volunteers and these patients and volunteers were classified into four groups: group 1, patients with active tuberculosis (n=30); group 2, patients with not-active tuberculosis (n=27); group 3, patients without tuberculosis (n=24); group 4, healthy volunteers without tuberculosis history (n=62). Positive rates and average spot counts of T-SPOT.TB test were obtained among these groups.
RESULTS
Positive rates of group 1 (96.4%) and group 2 (92.3%) were higher than those of group 3 (31.6%) and group 4 (27.4%) (P<0.0001). The sensitivity deduced from group 1 and specificity deduced from group 4 of T-SPOT.TB test were 96.4% and 72.6%, respectively. The average spot counts of group 1 and 2 were higher than those of group 3 and 4 (P<0.001). There was a tendency of increasing positive rate with increasing age. Overall agreement between T-SPOT.TB test and tuberculin skin test was 63.8% (kappa=0.29).
CONCLUSIONS
T-SPOT.TB test would be a very useful screening and supplementary test for diagnosis of tuberculosis due to its high sensitivity. However, positive results of T-SPOT.TB should be cautiously interpreted because of high positivity in treated tuberculosis patients and healthy volunteers in Korea.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Analysis of Patients with Positive Acid-fast Bacilli Culture and Negative T-SPOT.TB Results
You Mie Han, Hyun Soo Kim, Cheol Hong Kim, Hee Jung Kang, Kyu Man Lee
Korean J Lab Med. 2010;30(4):414-419. doi: 10.3343/kjlm.2010.30.4.414.
Reference
-
1.Lee JY., Shim TS. Diagnosis of mycobacterium tuberculosis infection using ex-vivo interferon-gamma assay. Tuberc Respir Dis. 2006. 60:497–509. (이정연및심태선. 체외 Interferon-gamma 검사를이용한결핵감염의진단. 결핵및호흡기질환 2006;60:497-509.).
Article2.Lee JY., Choi HJ., Park IN., Hong SB., Oh YM., Lim CM, et al. Comparison of two commercial interferon-gamma assays for diagnosing Mycobacterium tuberculosis infection. Eur Respir J. 2006. 28:24–30.3.Domínguez J., De Souza-Galvão M., Ruiz-Manzano J., Latorre I., Prat C., Lacoma A, et al. T-cell responses to the Mycobacterium tuberculosis-specific antigens in active tuberculosis patients at the beginning, during, and after antituberculosis treatment. Diagn Microbiol Infect Dis. 2009. 63:43–51.
Article4.Dye C., Scheele S., Dolin P., Pathania V., Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999. 282:677–86.5.World Health Organization. Tuberculosis. http://www.who.int/tb. (Updated on. 2009.6.Kang YA., Lee HW., Yoon HI., Cho B., Han SK., Shim YS, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005. 293:2756–61.7.Kang YA., Lee HW., Hwang SS., Um SW., Han SK., Shim YS, et al. Usefulness of whole-blood interferon-γ assay and interferon-γ enzyme-linked immunospot assay in the diagnosis of active pulmonary tuberculosis. Chest. 2007. 132:959–65.
Article8.American Thoracic Society. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000. 161:1376–95.9.Clinical and Laboratory Standards Institute. User protocol for evaluation of qualitative test performance; Approved guideline-Second edition. CLSI document EP12-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2008.10.Linnet K., Boyd JC. Selection and analytical evaluation of methods-with statistical technique. Burtis CA, Ashwood ER, editors. Tietz textbook of clinical chemistry and molecular diagnostics. 4th ed.St. Louis: Elsevier Saunders;2006. p. 367–8.11.Provinciali M., Moresi R., Donnini A., Lisa RM. Reference values for CD4+ and CD8+ T lymphocytes with naïve or memory phenotype and their association with mortality in the elderly. Gerontology. 2009. 55:314–21.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Patients with Positive Acid-fast Bacilli Culture and Negative T-SPOT.TB Results
- Discordance between Tuberculin Skin Test and Interferon-gamma Release Assays for Diagnosis of Tuberculosis Infection in Korean Children
- The Usefulness of In Vitro Interferon-gamma Assay for Differential Diagnosis between Intestinal Tuberculosis and Crohn's Disease
- Indeterminate T-SPOT.TB Test Results in Patients with Suspected Extrapulmonary Tuberculosis in Routine Clinical Practice
- Clinical Utility of Two Interferon-gamma Release Assays on Pleural Fluid for the Diagnosis of Tuberculous Pleurisy