Korean J Radiol.
2006 Dec;7(4):221-228. 10.3348/kjr.2006.7.4.221.
The Fate of High-Density Lesions on the Non-contrast CT Obtained Immediately After Intra-arterial Thrombolysis in Ischemic Stroke Patients
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. dhlee@amc.seoul.kr
- KMID: 1092544
- DOI: http://doi.org/10.3348/kjr.2006.7.4.221
Abstract
OBJECTIVE
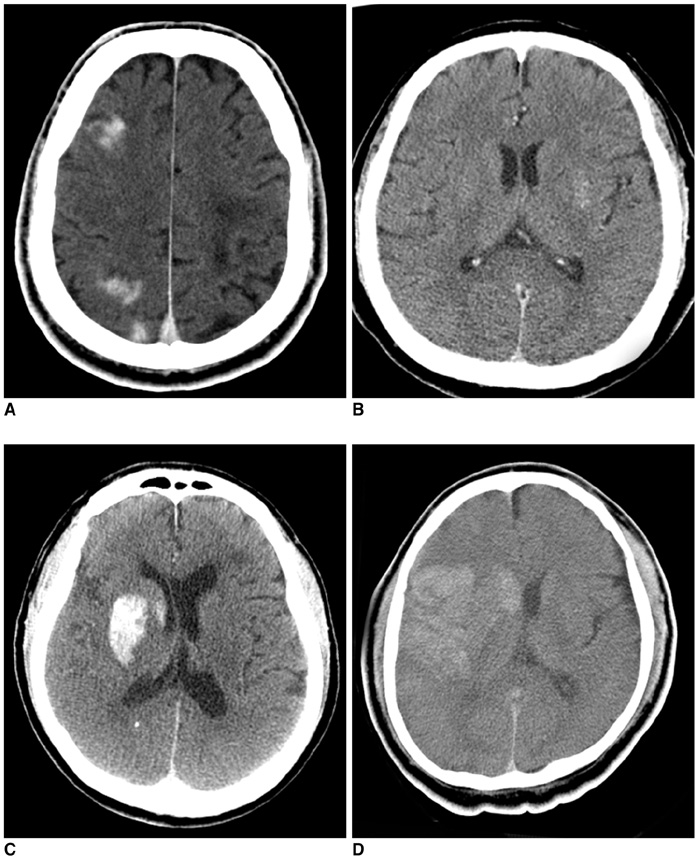
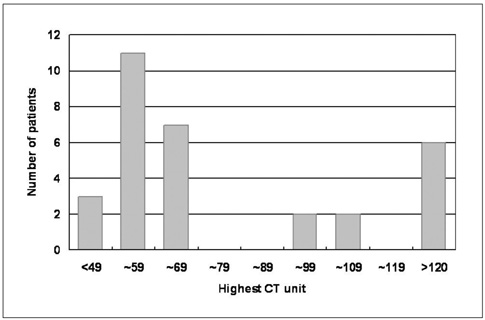
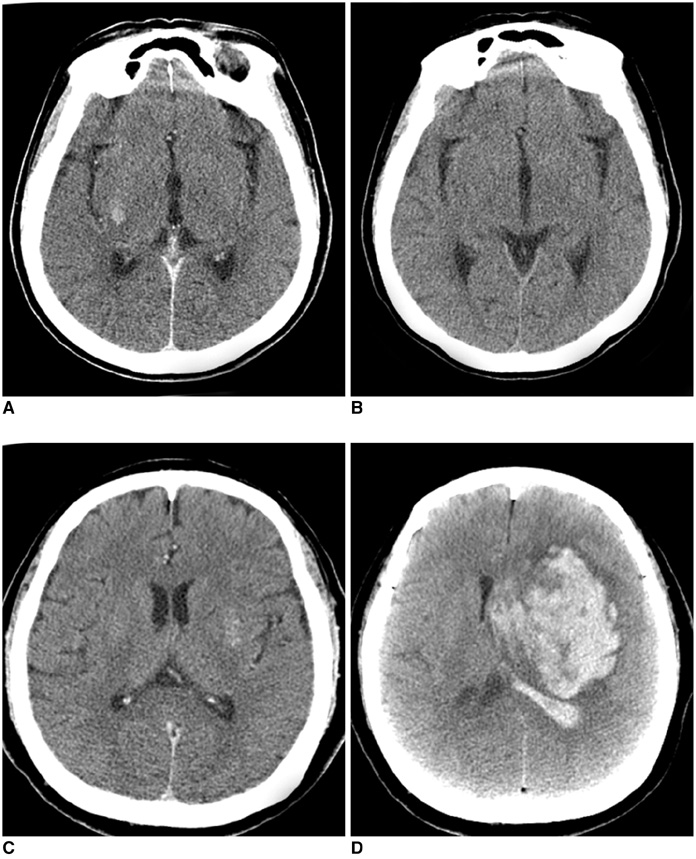
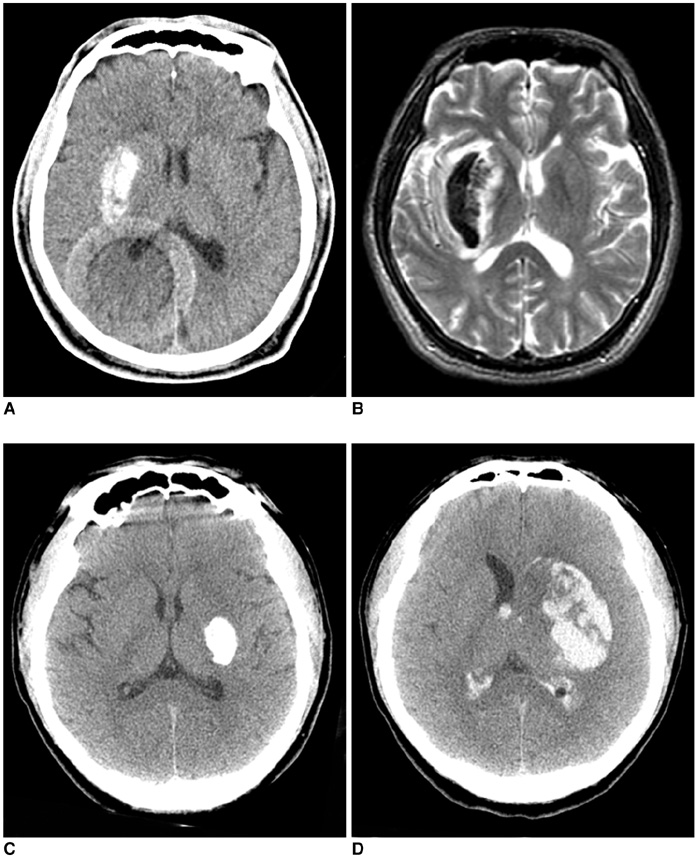
Hyperdense lesions can frequently be observed on the CT obtained immediately after intra-arterial (IA) thrombolysis, and it is sometimes difficult to differentiate contrast extravasation from the hemorrhagic lesions. The purposes of this study are to classify the hyperdense lesions according to their morphologic features and to track the outcome of those lesions. MATERIALS AND METHODS: Among the 94 patients who suffered with anterior circulation ischemic stroke and who were treated with IA thrombolysis, 31 patients revealed hyperdense lesions on the CT obtained immediately after the procedure. The lesions were categorized into four types according to their volume, shape, location and density: cortical high density (HD), soft HD, metallic HD and diffuse HD. The follow-up images were obtained 3-5 days later in order to visualize the morphologic changes and hemorrhagic transformation of the lesions. RESULTS: Among the 31 patients with HD lesions, 18 (58%) showed hemorrhagic transformation of their lesion, and six of them were significant. All the cortical HD lesions (n = 4) revealed spontaneous resolution. Seven of the soft HD lesions (n = 13) showed spontaneous resolution, while the rest of the group showed hemorrhagic transformation. Among them the hemorrhage was significant in only two patients (2/6) who did not achieve successful recanalization. All the metallic HD lesions (n = 10) resulted in hemorrhagic transformation; among them, three cases (30%) with a maximum CT value more than 150 HU (Hounsfield unit) subsequently showed significant hemorrhagic transformation on the follow-up CT. There were four diffuse HD lesions, and two of them showed hemorrhagic transformation. CONCLUSION: The parenchymal hyperdense lesions observed on the CT obtained immediately after IA thrombolysis in ischemic stroke patients exhibited varying features and they were not always hemorrhagic. Most of the soft HD lesions were benign, and although all of the metallic HD lesions were hemorrhagic, some of them were ultimately found to be benign.
MeSH Terms
-
Treatment Outcome
*Tomography, X-Ray Computed
Thrombolytic Therapy/*methods
Statistics, Nonparametric
Retrospective Studies
Middle Aged
Male
Magnetic Resonance Imaging
Infusions, Intra-Arterial
Humans
Fibrinolytic Agents/therapeutic use
Female
Cerebrovascular Accident/*drug therapy/pathology/*radiography
Brain Ischemia/*drug therapy/pathology/*radiography
Aged
Acute Disease
Figure
Reference
-
1. Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. Jama. 1999. 282:2003–2011.2. Higashida R, Furlan A, Roberts H, Tomsick T, Connors B, Barr J, et al. Trial design and reporting standards for intraarterial cerebral thrombolysis for acute ischemic stroke. J Vasc Interv Radiol. 2003. 14:S493–S494.3. Nakano S, Iseda T, Kawano H, Yoneyama T, Ikeda T, Wakisaka S. Parenchymal hyperdensity on computed tomography after intra-arterial reperfusion therapy for acute middle cerebral artery occlusion: incidence and clinical significance. Stroke. 2001. 32:2042–2048.4. Wildenhain SL, Jungreis CA, Barr J, Mathis J, Wechsler L, Horton JA. CT after intracranial intraarterial thrombolysis for acute stroke. AJNR Am J Neuroradiol. 1994. 15:487–492.5. Mericle RA, Lopes DK, Fronckowiak MD, Wakhloo AK, Guterman LR, Hopkins LN. A grading scale to predict outcomes after intra-arterial thrombolysis for stroke complicated by contrast extravasation. Neurosurgery. 2000. 46:1307–1314. discussion 1314-1315.6. Yoon W, Seo JJ, Kang HK. Bilateral paramedian thalamic contrast enhancement on CT after Intra-arterial thrombolysis. Korean J Radiol. 2005. 6:41–43.7. Komiyama M, Nishijima Y, Nishio A, Khosla VK. Extravasation of contrast medium from the lenticulostriate artery following local intracarotid fibrinolysis. Surg Neurol. 1993. 39:315–319.8. Yoon W, Seo JJ, Kim JK, Cho KH, Park JG, Kang HK. Contrast enhancement and contrast extravasation on computed tomography after intra-arterial thrombolysis in patients with acute ischemic stroke. Stroke. 2004. 35:876–881.9. Yokogami K, Nakano S, Ohta H, Goya T, Wakisaka S. Prediction of hemorrhagic complications after thrombolytic therapy for middle cerebral artery occlusion: value of pre- and post-therapeutic computed tomographic findings and angiographic occlusive site. Neurosurgery. 1996. 39:1102–1107.10. del Zoppo GJ, von Kummer R, Hamann GF. Ischaemic damage of brain microvessels: inherent risks for thrombolytic treatment in stroke. J Neurol Neurosurg Psychiatry. 1998. 65:1–9.11. del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003. 23:879–894.12. Dijkhuizen RM, Asahi M, Wu O, Rosen BR, Lo EH. Rapid breakdown of microvascular barriers and subsequent hemorrhagic transformation after delayed recombinant tissue plasminogen activator treatment in a rat embolic stroke model. Stroke. 2002. 33:2100–2104.13. Hacke W, Donnan G, Fieschi C, Kaste M, von Kummer R, Broderick JP, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004. 363:768–774.14. Berger C, Fiorelli M, Steiner T, Schabitz WR, Bozzao L, Bluhmki E, et al. Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic? Stroke. 2001. 32:1330–1335.15. Adams HP Jr, Adams RJ, Brott T, del Zoppo GJ, Furlan A, Goldstein LB, et al. Guidelines for the early management of patients with ischemic stroke: A scientific statement from the Stroke Council of the American Stroke Association. Stroke. 2003. 34:1056–1083.16. Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003. 34:2025–2030.17. Broderick JP, Hacke W. Treatment of acute ischemic stroke: Part II: neuroprotection and medical management. Circulation. 2002. 106:1736–1740.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bilateral Paramedian Thalamic Contrast Enhancement on CT after Intra-arterial Thrombolysis
- Significance of High Density Lesion on Immediate follow-up CT scan after Intraarterial Thrombolysis for Acute Ischemic Stroke
- Change of Apparent Diffusion Coefficient Immediately after Recanalization through Intra-Arterial Revascularization Therapy in Acute Ischemic Stroke
- Intra-arterial Thrombolysis for Central Retinal Artery Occlusion after the Coil Embolization of Paraclinoid Aneurysm
- Recent advances in thrombolysis of acute ischemic stroke