Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs
- Affiliations
-
- 1Department of Veterinary Surgery, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. ohkweon@snu.ac.kr
- 2Laboratory of Stem Cell and Tumor Biology, Department of Veterinary Public Health, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. kangpub@snu.ac.kr
- 3College of Veterinary Medicine, Research Institute of Veterinary Medicine, Chungnam National University, Daejeon 305-764, Korea.
- KMID: 1090803
- DOI: http://doi.org/10.4142/jvs.2007.8.3.275
Abstract
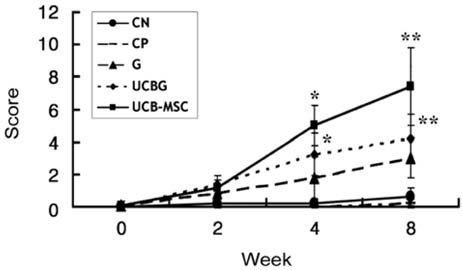
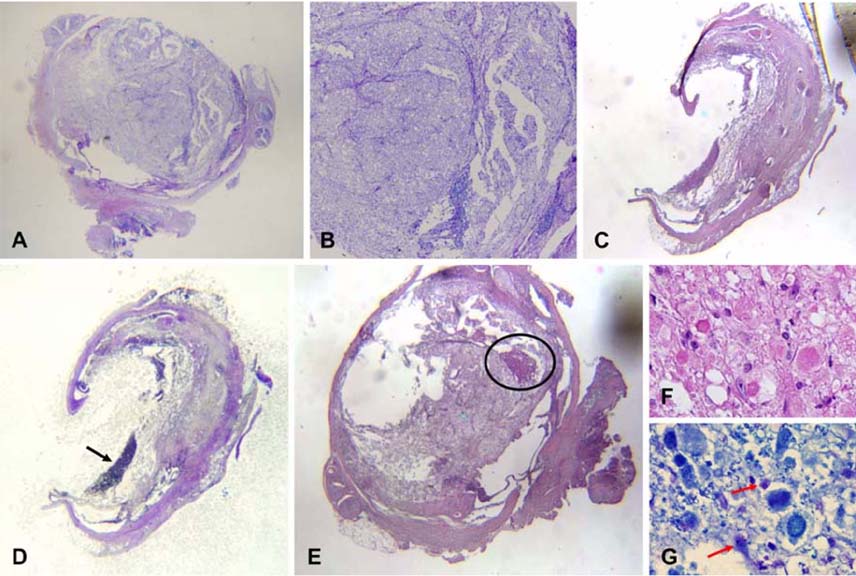
- This study was to determine the effects of allogenicumbilical cord blood (UCB)-derived mesenchymal stemcells (MSCs) and recombinant methionyl humangranulocyte colony-stimulating factor (rmhGCSF) on acanine spinal cord injury model after balloon compressionat the first lumbar vertebra. Twenty-five adult mongreldogs were assigned to five groups according to treatmentafter a spinal cord injury: no treatment (CN); salinetreatment (CP); rmhGCSF treatment (G); UCB-MSCstreatment (UCB-MSC); co-treatment (UCBG). The UCB-MSCs isolated from cord blood of canine fetuses wereprepared as 10(6) cells/150microl saline. The UCB-MSCs weredirectly injected into the injured site of the spinal cord andrmhGCSF was administered subcutaneously 1 week afterthe induction of spinal cord injury. The Olby score,magnetic resonance imaging, somatosensory evokedpotentials and histopathological examinations were used toevaluate the functional recovery after transplantation. TheOlby scores of all groups were zero at the 0-week evaluation.At 2 week after the transplantation, the Olby scores in thegroups with the UCB-MSC and UCBG were significantlyhigher than in the CN and CP groups. However, there wereno significant differences between the UCB-MSC andUCBG groups, and between the CN and CP groups. Thesecomparisons remained stable at 4 and 8 week aftertransplantation. There was significant improvement in thenerve conduction velocity based on the somatosensory evokedpotentials. In addition, a distinct structural consistency ofthe nerve cell bodies was noted in the lesion of the spinalcord of the UCB-MSC and UCBG groups. These resultssuggest that transplantation of the UCB-MSCs resulted inrecovery of nerve function in dogs with a spinal cord injuryand may be considered as a therapeutic modality for spinalcord injury.
MeSH Terms
-
Animals
Behavior, Animal/physiology
Cord Blood Stem Cell Transplantation/methods/*veterinary
Dog Diseases/pathology/*therapy
Dogs
Evoked Potentials, Somatosensory/physiology
Histocytochemistry/veterinary
Magnetic Resonance Imaging/veterinary
Random Allocation
Spinal Cord Injuries/pathology/therapy/*veterinary
Videotape Recording
Figure
Cited by 10 articles
-
Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury
Hak-Hyun Ryu, Ji-Hey Lim, Ye-Eun Byeon, Jeong-Ran Park, Min-Soo Seo, Young-Won Lee, Wan Hee Kim, Kyung-Sun Kang, Oh-Kyeong Kweon
J Vet Sci. 2009;10(4):273-284. doi: 10.4142/jvs.2009.10.4.273.Immunologic properties of differentiated and undifferentiated mesenchymal stem cells derived from umbilical cord blood
Hyo-Jong Lee, Kyung-Sun Kang, Sun-Young Kang, Hyung-Sik Kim, Se-Jin Park, Seung-Yong Lee, Kwang-Dong Kim, Hee-Chun Lee, Ji-Kwon Park, Won-Young Paik, Lyon Lee, Seong-Chan Yeon
J Vet Sci. 2016;17(3):289-297. doi: 10.4142/jvs.2016.17.3.289.Expression of neurotrophic factors in injured spinal cord after transplantation of human-umbilical cord blood stem cells in rats
Hyo-jin Chung, Wook-hun Chung, Jae-Hoon Lee, Dai-Jung Chung, Wo-Jong Yang, A-Jin Lee, Chi-Bong Choi, Hwa-Seok Chang, Dae-Hyun Kim, Hyun Jung Suh, Dong-Hun Lee, Soo-Han Hwang, Sun Hee Do, Hwi-Yool Kim
J Vet Sci. 2016;17(1):97-102. doi: 10.4142/jvs.2016.17.1.97.Effect of canine mesenchymal stromal cells overexpressing heme oxygenase-1 in spinal cord injury
Seung Hoon Lee, Yongsun Kim, Daeun Rhew, Ahyoung Kim, Kwang Rae Jo, Yongseok Yoon, Kyeung Uk Choi, Taeseong Jung, Wan Hee Kim, Oh-Kyeong Kweon
J Vet Sci. 2017;18(3):377-386. doi: 10.4142/jvs.2017.18.3.377.Implantation of canine umbilical cord blood-derived mesenchymal stem cells mixed with beta-tricalcium phosphate enhances osteogenesis in bone defect model dogs
Byung-Jun Jang, Ye-Eun Byeon, Ji-Hey Lim, Hak-Hyun Ryu, Wan Hee Kim, Yoshihisa Koyama, Masanori Kikuchi, Kyung-Sun Kang, Oh-Kyeong Kweon
J Vet Sci. 2008;9(4):387-393. doi: 10.4142/jvs.2008.9.4.387.Effect of subcutaneous treatment with human umbilical cord blood-derived multipotent stem cells on peripheral neuropathic pain in rats
Min Ju Lee, Tae Gyoon Yoon, Moonkyu Kang, Hyun Jeong Kim, Kyung Sun Kang
Korean J Physiol Pharmacol. 2017;21(2):153-160. doi: 10.4196/kjpp.2017.21.2.153.Changes of the Electrophysiological Study in Dogs with Acute Spinal Cord Injury
Joongkee Min, Ji Yun Kim, Cheong Hoon Seo, Sang Ryong Jeon, Kyoung Hyo Choi, Je Hoon Jeong
Korean J Neurotrauma. 2014;10(1):1-5. doi: 10.13004/kjnt.2014.10.1.1.Stem Cell Treatment for Complicated Diabetes
Jong Yoon Bahk, Hoon Han, Youn Soo Lee
Int J Stem Cells. 2008;1(1):91-95.Stem Cell Treatment for Complicated Diabetes
Jong Yoon Bahk, Hoon Han, Youn Soo Lee
Int J Stem Cells. 2008;1(1):91-95.Is There Additive Therapeutic Effect When GCSF Combined with Adipose-Derived Stem Cell in a Rat Model of Acute Spinal Cord Injury?
Joongkee Min, Jeong Hoon Kim, Kyoung Hyo Choi, Hyung Ho Yoon, Sang Ryong Jeon
J Korean Neurosurg Soc. 2017;60(4):404-416. doi: 10.3340/jkns.2016.1010.008.
Reference
-
1. Akiyama Y, Radtke C, Kocsis JD. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J Neurosci. 2002. 22:6623–6630.
Article2. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002. 99:3838–3843.
Article3. Enomoto M, Wakabayashi Y, Qi ML, Shinomiya K. Present situation and future aspects of spinal cord regeneration. J Orthop Sci. 2004. 9:108–112.
Article4. Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000. 109:235–242.
Article5. Fine A. Transplantation of fetal cells and tissue: an overview. CMAJ. 1994. 151:1261–1268.6. Fukuda S, Nakamura T, Kishigami Y, Endo K, Azuma T, Fujikawa T, Tsutsumi S, Shimizu Y. New canine spinal cord injury model free from laminectomy. Brain Res Brain Res Protoc. 2005. 14:171–180.
Article7. Gang EJ, Hong SH, Jeong JA, Hwang SH, Kim SW, Yang IH, Ahn C, Han H, Kim H. In vitro mesengenic potential of human umbilical cord blood-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2004. 321:102–108.
Article8. Goodwin HS, Bicknese AR, Chien SN, Bogucki BD, Oliver DA, Quinn CO, Wall DA. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol Blood Marrow Transplant. 2001. 7:581–588.
Article9. Horky LL, Galimi F, Gage FH, Horner PJ. Fate of endogenous stem/progenitor cells following spinal cord injury. J Comp Neurol. 2006. 498:525–538.
Article10. Jendelová P, Herynek V, Urdzíková L, Glogarová K, Kroupová J, Andersson B, Bryja V, Burian M, Hájek M, Syková E. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res. 2004. 76:232–243.
Article11. Jeong YH, Lim JH, Jung CS, Kweon OK, Lee YS, Kang KS. Osteogenic potential of canine cord blood derived mesenchymal stem cell. 4th ISSCR Annual Meeting. 2006. Toronto: ISSCR;217.12. Jung KH, Chu K, Lee ST, Kim SJ, Sinn DI, Kim SU, Kim M, Roh JK. Granulocyte colony-stimulating factor stimulates neurogenesis via vascular endothelial growth factor with STAT activation. Brain Res. 2006. 1073-1074:190–201.
Article13. Kang KS, Kim SW, Oh YH, Yu JW, Kim KY, Park HK, Song CH, Han H. A 37-year-old spinal cord-injured female patient, transplanted of multipotent stem cells from human UC blood, with improved sensory perception and mobility, both functionally and morphologically: a case study. Cytotherapy. 2005. 7:368–373.
Article14. Komine-Kobayashi M, Zhang N, Liu M, Tanaka R, Hara H, Osaka A, Mochizuki H, Mizuno Y, Urabe T. Neuroprotective effect of recombinant human granulocyte colony-stimulating factor in transient focal ischemia of mice. J Cereb Blood Flow Metab. 2006. 26:402–413.
Article15. Kuh SU, Cho YE, Yoon DH, Kim KN, Ha Y. Functional recovery after human umbilical cord blood cells transplantation with brain-derived neutrophic factor into the spinal cord injured rat. Acta Neurochir (Wien). 2005. 147:985–992.
Article16. Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003. 31:890–896.
Article17. Lee J, Nam T. Evaluation of spinal cord dysfunction by the somatosensory evoked potentials in dogs [Ph.D dissertation]. 2000. Seoul: Seoul National University;66–69.18. Lee ST, Chu K, Jung KH, Ko SY, Kim EH, Sinn DI, Lee YS, Lo EH, Kim M, Roh JK. Granulocyte colony-stimulating factor enhances angiogenesis after focal cerebral ischemia. Brain Res. 2005. 1058:120–128.
Article19. Lim JH, Jung CS, Byeon YE, Kim WH, Yoon JH, Kang KS, Kweon OK. Establishment of a canine spinal cord injury model induced by epidural balloon compression. J Vet Sci. 2007. 8:89–94.
Article20. Lu D, Sanberg PR, Mahmood A, Li Y, Wang L, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant. 2002. 11:275–281.
Article21. Mautes AEM, Liu J, Brandewiede J, Manville J, Snyder E, Schachner M. Regional energy metabolism following short-term neural stem cell transplantation into the injured spinal cord. J Mol Neurosci. 2004. 24:227–236.
Article22. Nakage APM, Santana AE, de Cápua MLB, Godoy AV. Characterization and quantification of blood cells from the umbilical cord of dogs. Vet Clin Pathol. 2005. 34:394–396.
Article23. Olby N, Harris T, Burr J, Muñana K, Sharp N, Keene B. Recovery of pelvic limb function in dogs following acute intervertebral disc herniations. J Neurotrauma. 2004. 21:49–59.
Article24. Olby NJ, De Risio L, Muñana KR, Wosar MA, Skeen TM, Sharp NJ, Keene BW. Development of a functional scoring system in dogs with acute spinal cord injuries. Am J Vet Res. 2001. 62:1624–1628.
Article25. Park HC, Shim YS, Ha Y, Yoon SH, Park SR, Choi BH, Park HS. Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Eng. 2005. 11:913–922.
Article26. Park KS, Lee YS, Kang KS. In vitro neuronal and osteogenic differentiation of mesenchymal stem cells from human umbilical cord blood. J Vet Sci. 2006. 7:343–348.
Article27. Pluchino S, Zanotti L, Deleidi M, Martino G. Neural stem cells and their use as therapeutic tool in neurological disorders. Brain Res Brain Res Rev. 2005. 48:211–219.
Article28. Poncelet L, Michaux C, Balligand M. Somatosensory potentials in dogs with naturally acquired thoracolumbar spinal cord disease. Am J Vet Res. 1993. 54:1935–1941.29. Poncelet L, Michaux C, Balligand M. Study of spinal cord evoked injury potential by use of computer modeling and in dogs with naturally acquired thoracolumbar spinal cord compression. Am J Vet Res. 1998. 59:300–306.30. Rogers I, Casper RF. Umbilical cord blood stem cells. Best Pract Res Clin Obstet Gynaecol. 2004. 18:893–908.
Article31. Safford KM, Rice HE. Stem cell therapy for neurologic disorders: therapeutic potential of adipose-derived stem cells. Curr Drug Targets. 2005. 6:57–62.
Article32. Satake K, Lou J, Lenke LG. Migration of mesenchymal stem cells through cerebrospinal fluid into injured spinal cord tissue. Spine. 2004. 29:1971–1979.
Article33. Schultz SS. Adult stem cell application in spinal cord injury. Curr Drug Targets. 2005. 6:63–73.
Article34. Suter SE, Gouthro TA, McSweeney PA, Nash RA, Haskins ME, Felsburg PJ, Henthorn PS. Isolation and characterization of pediatric canine bone marrow CD34+ cells. Vet Immunol Immunopathol. 2004. 101:31–47.
Article35. Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci USA. 2002. 99:9606.
Article36. van Os R, Robinson S, Sheridan T, Mauch PM. Granulocyte-colony stimulating factor impedes recovery from damage caused by cytotoxic agents through increased differentiation at the expense of self-renewal. Stem Cells. 2000. 18:120–127.
Article37. Watt SM, Contreras M. Stem cell medicine: umbilical cord blood and its stem cell potential. Semin Fetal Neonatal Med. 2005. 10:209–220.
Article38. Webb AA, Jeffery ND, Olby NJ, Muir GD. Behavioural analysis of the efficacy of treatments for injuries to the spinal cord in animals. Vet Rec. 2004. 155:225–230.
Article39. Yang JW, Jeong SM, Seo KM, Nam TC. Effects of corticosteroid and electroacupuncture on experimental spinal cord injury in dogs. J Vet Sci. 2003. 4:97–101.
Article40. Zhao ZM, Li HJ, Liu HY, Lu SH, Yang RC, Zhang QJ, Han ZC. Intraspinal transplantation of CD34+ human umbilical cord blood cells after spinal cord hemisection injury improves functional recovery in adult rats. Cell Transplant. 2004. 13:113–122.
Article41. Zhu J, Wu X, Zhang HL. Adult neural stem cell therapy: expansion in vitro, tracking in vivo and clinical transplantation. Curr Drug Targets. 2005. 6:97–110.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Percutaneous transplantation of human umbilical cord-derived mesenchymal stem cells in a dog suspected to have fibrocartilaginous embolic myelopathy
- Umbilical Cord Blood Transplantation
- Stem Cell Transplantation in Umbilical Cord Blood(I) Expansion Effects of Stem Cells in Umbilical Cord Blood with Various Hematopoietic Growth Factors
- Use of Cord Blood Stem Cells in Cell Therapy
- ERRATUM: Isolation and characterization of canine umbilical cord blood-derived mesenchymal stem cells