J Vet Sci.
2007 Jun;8(2):163-168. 10.4142/jvs.2007.8.2.163.
Hematology, cytochemistry and ultrastructure of blood cells in fishing cat (Felis viverrina)
- Affiliations
-
- 1Department of Pathology, Faculty of Veterinary Medicine, Kasetsart University, Bangkok-10900, Thailand. fvetksp@ku.ac.th
- 2Department of Pathology, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok-10400, Thailand.
- 3Central Instrumentation Unit, Faculty of Science, Mahasarakarm University, Mahasarakarm-44150, Thailand.
- KMID: 1089668
- DOI: http://doi.org/10.4142/jvs.2007.8.2.163
Abstract
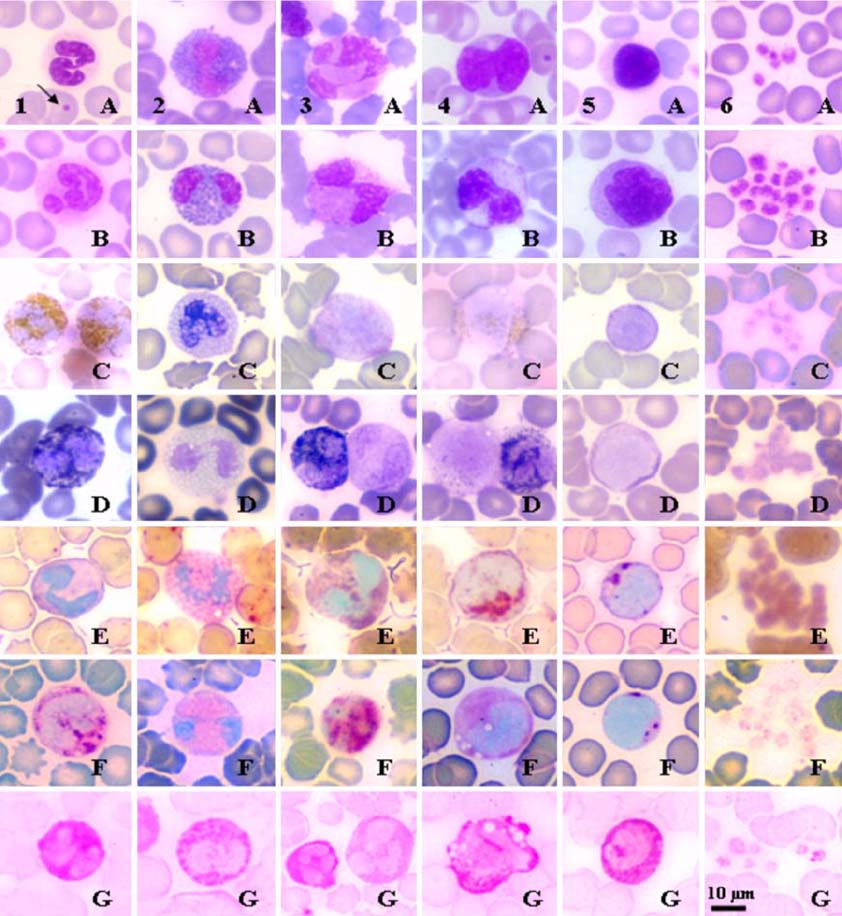
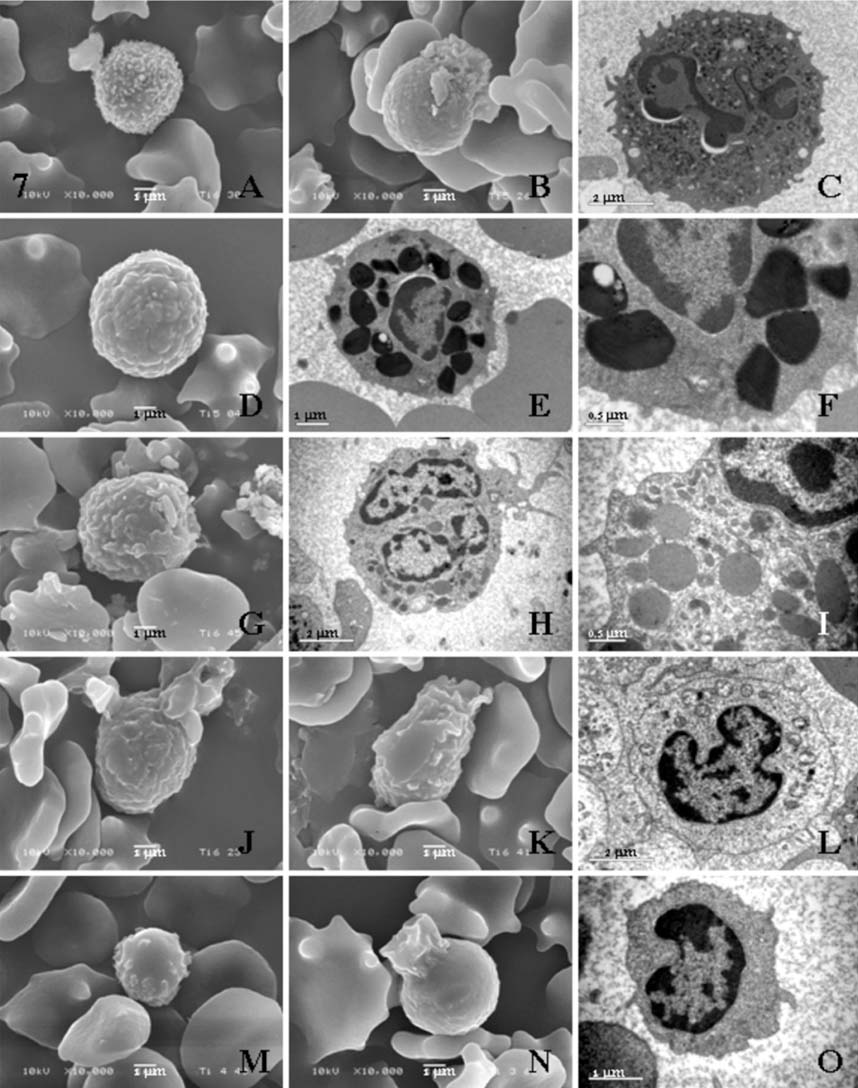
- Hematological, cytochemical and ultrastructural features of blood cells in fishing cat (Felis viverrina) were evaluated using complete blood cell counts with routine and cytochemical blood stains, and scanning and transmission electron microscopy. No statistically significant difference was found in different genders of this animal. Unique features of blood cells in this animal were identified in hematological, cytochemical and ultrastructural studies. This study contributes to broaden hematological resources in wildlife animals and provides a guideline for identification of blood cells in the fishing cat.
Keyword
MeSH Terms
Figure
Reference
-
1. Clinkenbeard KD, Meinkoth JH. Feldman BF, Zinkl JG, Jain NC, editors. Normal hematology of the cat. Schalm's Veterinary Hematology. 2000. 5th ed. Philadelphia: Lippincott Williams & Wilkins;1064–1068.2. Hayhoe FGJ, Quaglino D. Haematological Cytochemistry. 1980. Edinburgh: Churchill Livingstone;68–75.3. Jain NC. Essentials of Veterinary Hematology. 1993. Philadelphia: Lea & Febiger;417.4. Jain NC. Schalm's Veterinary Hematology. 1986. 4th ed. Philadelphia: Lea & Febiger;1221.5. Kaplow LS. Simplified myeloperoxidase stain using benzidine dihydrochloride. Blood. 1965. 26:215–219.6. Lekagul B, McNeely JA, editors. Family felidae. Mammals of Thailand. 1988. 2nd ed. Bangkok: Darnsutha Press;603–630.7. Raskin RE, Valenciano A. Feldman BF, Zinkl JG, Jain NC, editors. Cytochemistry of normal leukocytes. Schalm's Veterinary Hematology. 2000. 5th ed. Philadelphia: Lippincott Williams & Wilkins;337–346.8. Reagan WJ, Sanders TG, DeNicola DB. Veterinary Hematology: Atlas of Common Domestic Species. 1998. Ames: Manson Publishing;75.9. Salakij C, Salakij J, Apibal S, Narkkong NA, Chanhome L, Rochanapat N. Hematology, morphology, cytochemical staining, and ultrastructural characteristics of blood cells in king cobras (Ophiophagus hannah). Vet Clin Pathol. 2002. 31:116–126.
Article10. Salakij C, Salakij J, Narkkong NA, Trongwonsa L, Pattanarangsan R. Hematology, cytochemistry and ultrastructure of blood cells from Asiatic black bear (Ursus thibetanus). Kasetsart J (Nat Sci). 2005. 39:247–261.11. Salakij J, Salakij C, Narkkong NA, Apibal S, Suthunmapinuntra P, Rattanakunuprakarn J, Nunklang G, Yindee M. Hematology, cytochemistry and ultrastructure of blood cells from Asian elephant (Elephas maximus). Kasetsart J (Nat Sci). 2005. 39:482–493.12. Sheehan HL, Storey GW. An improved method of staining leukocyte granules with Sudan black B. J Pathol Bacteriol. 1947. 59:336–337.13. Steffens WL. Feldman BF, Zinkl JG, Jain NC, editors. Ultrastructure features of leukocytes. Schalm's Veterinary Hematology. 2000. 5th ed. Philadelphia: Lippincott Williams & Wilkins;326–336.14. Tablin F. Feldman BF, Zinkl JG, Jain NC, editors. Platelet Structure and Function. Schalm's Veterinary Hematology. 2000. 5th ed. Philadelphia: Lippincott Williams & Wilkins;448–452.15. Tsujimoto H, Hasegawa A, Tomoda I. A cytochemical study on feline blood cells. Nippon Juigaku Zasshi. 1983. 45:373–382.
Article16. Yam LT, Li CY, Crosby WH. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971. 55:283–290.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Acute monoblastic leukemia in a FeLV-positive cat
- Surface ultrastructure of the adult stage of Acanthotrema felis (Trematoda: Heterophyidae
- Evaluation of assays to detect Helicobacter felis infection in cats
- Detection of Helicobacter felis in a cat with gastric disease in laboratory animal facility
- Investigation of chlamydophilosis from naturally infected cats