J Vet Sci.
2011 Sep;12(3):267-272. 10.4142/jvs.2011.12.3.267.
Transmission electron microscopy for characterization of acrosomal damage after Percoll gradient centrifugation of cryopreserved bovine spermatozoa
- Affiliations
-
- 1Department of Preventive Veterinary Medicine and Animal Reproduction, School of Agrarian Sciences and Veterinary Medicine, Universidade Estadual Paulista, Jaboticabal 14884-900, Brazil. leticiazoccolaro@yahoo.com.br
- 2Laboratory of Histology, Department of Morphology, Biomedical Sciences Institute, Universidade Federal de Uberlandia, Uberlandia 38400-902, Brazil.
- 3Department of Animal Reproduction, School of Veterinary Medicine, Universidade Federal de Uberlandia, Uberlandia 38400-902, Brazil.
- 4Laboratory of Semen Biotechnology and Andrology, Department of Animal Reproduction, Veterinary Medicine and Animal Science School, Universidade de Sao Paulo, Pirassununga 13630-000, Brazil.
- KMID: 1067399
- DOI: http://doi.org/10.4142/jvs.2011.12.3.267
Abstract
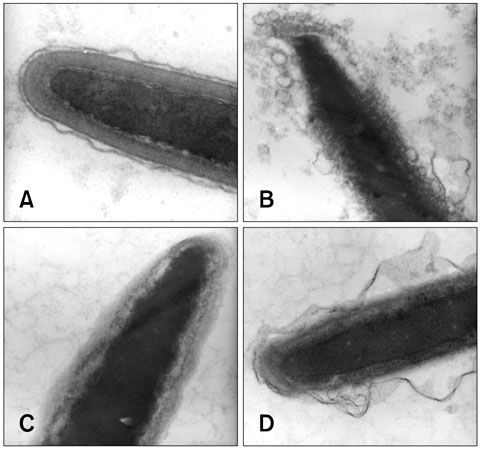
- The objective of this study was to characterize acrosomal ultrastructure following discontinuous Percoll gradient centrifugation of cryopreserved bovine sperm. Semen was collected from six bulls of different breeds and three ejaculates per bull were evaluated. Frozen semen samples were thawed and the acrosomal region of sperm cells was evaluated by transmission electron microscopy (TEM) before (n = 18) and after (n = 18) Percoll centrifugation. The evaluation of 20 sperm heads from each of the 36 samples analyzed ensured that a large number of cells were investigated. The data were subjected to analysis of variance at a level of significance of 5%. Percoll centrifugation reduced the percentage of sperm exhibiting normal acrosomes (from 61.77 to 30.24%), reduced the percentage of sperm presenting atypical acrosome reactions (from 28.38 to 4.84%) and increased the percentage of sperm exhibiting damage in the acrosome (from 6.14 to 64.26%). The percentage of sperm with typical acrosome reactions was not significantly different before (3.70%) and after (0.67%) centrifugation. TEM distinguished four different types of acrosomal status and enabled ultrastructural characterization of acrosomal injuries. The percentage of sperm exhibiting normal acrosomes decreased and damage in the acrosome was the most frequent acrosomal injury with the Percoll gradient centrifugation protocol utilized.
Keyword
MeSH Terms
-
Acrosome/*pathology/ultrastructure
Animals
Cattle/*physiology
Cell Membrane/*pathology/ultrastructure
Cell Separation/veterinary
Centrifugation, Density Gradient/veterinary
Cryopreservation/veterinary
Male
Microscopy, Electron, Transmission/veterinary
Povidone/*adverse effects
Silicon Dioxide/*adverse effects
Spermatozoa/pathology/ultrastructure
Figure
Reference
-
1. Aitken RJ, Clarkson JS. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J Androl. 1988. 9:367–376.
Article2. Arcidiacono A, Walt H, Campana A, Balerna M. The use of Percoll gradients for the preparation of subpopulations of human spermatozoa. Int J Androl. 1983. 6:433–445.
Article3. Celeghini ECC, de Arruda RP, de Andrade AFC, Nascimento J, Raphael CF. Practical techniques for bovine sperm simultaneous fluorimetric assessment of plasma, acrosomal and mitochondrial membranes. Reprod Domest Anim. 2007. 42:479–488.
Article4. Cesari A, Kaiser GG, Mucci N, Mutto A, Vincenti A, Fornés MW, Alberio RH. Integrated morphophysiological assessment of two methods for sperm selection in bovine embryo production in vitro. Theriogenology. 2006. 66:1185–1193.
Article5. Cross NL, Meizel S. Methods for evaluating the acrosomal status of mammalian sperm. Biol Reprod. 1989. 41:635–641.6. Cross NL, Morales P, Overstreet JW, Hanson FW. Two simple methods for detecting acrosome-reacted human sperm. Gamete Res. 1986. 15:213–226.
Article7. Dode MAN, Rodovalho NC, Ueno VG, Fernandes CE. The effect of sperm preparation and co-incubation time on in vitro fertilization of Bos indicus oocytes. Anim Reprod Sci. 2002. 69:15–23.
Article8. Flesch FM, Gadella BM. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim Biophys Acta. 2000. 1469:197–235.
Article9. Holt WV. Basic aspects of frozen storage of semen. Anim Reprod Sci. 2000. 62:3–22.
Article10. Le Lannou D, Blanchard Y. Nuclear maturity and morphology of human spermatozoa selected by Percoll density gradient centrifugation or swim-up procedure. J Reprod Fertil. 1988. 84:551–556.
Article11. Lu KH, Seidel GE Jr. Effects of heparin and sperm concentration on cleavage and blastocyst development rates of bovine oocytes inseminated with flow cytometrically-sorted sperm. Theriogenology. 2004. 62:819–830.
Article12. Machado GM, Carvalho JO, Filho ES, Caixeta ES, Franco MM, Rumpf R, Dode MAN. Effect of Percoll volume, duration and force of centrifugation, on in vitro production and sex ratio of bovine embryos. Theriogenology. 2009. 71:1289–1297.
Article13. McCann CT, Chantler E. Properties of sperm separated using Percoll and IxaPrep density gradients. A comparison made using CASA, longevity, morphology and the acrosome reaction. Int J Androl. 2000. 23:205–209.
Article14. Mehmood A, Anwar M, Saqlan Naqvi SM. Motility, acrosome integrity, membrane integrity and oocyte cleavage rate of sperm separated by swim-up or Percoll gradient method from frozen-thawed buffalo semen. Anim Reprod Sci. 2009. 111:141–148.
Article15. Morrell JM, Rodriguez-Martinez H. Biomimetic techniques for improving sperm quality in animal breeding: a review. Open Androl J. 2009. 1:1–9.16. Oliveira LZ, Arruda RP, Celeghini ECC, de Andrade AFC, Perini AP, Resende MV, Miguel MCV, Lucio AC, Hossepian de Lima VFM. Effects of discontinuous Percoll gradient centrifugation on the quality of bovine spermatozoa evaluated with computer-assisted semen analysis and fluorescent probes association. Andrologia. 2011. doi: 10.1111/j.1439-0272.2010.01096.x. [Epub ahead of print].
Article17. Oshio S. Apparent densities of spermatozoa of various mammalian species. Gamete Res. 1988. 20:159–164.
Article18. Öura C, Toshimori K. Ultrastructural studies on the fertilization of mammalian gametes. Int Rev Cytol. 1990. 122:105–151.19. Petyim S, Choavaratana R, Suksompong S, Laokirkkiat P, Makemaharn O. Outcome of sperm preparation using double-gradients technique study in siriraj hospital. J Med Assoc Thai. 2009. 92:878–884.20. Rhemrev J, Jeyendran RS, Vermeiden JP, Zaneveld LJ. Human sperm selection by glass wool filtration and two-layer, discontinuous Percoll gradient centrifugation. Fertil Steril. 1989. 51:685–690.
Article21. Saacke RG, Marshall CE. Observations on the acrosomal cap of fixed and unfixed bovine spermatozoa. J Reprod Fertil. 1968. 16:511–514.
Article22. Somfai T, Bodó S, Nagy S, Papp ÁB, Iváncsics J, Baranyai B, Gócza E, Kovács A. Effect of Swim up and Percoll treatment on viability and acrosome integrity of frozen-thawed bull spermatozoa. Reprod Domest Anim. 2002. 37:285–290.
Article23. Sterzik K, De Santo M, Uhlich S, Gagsteiger F, Strehler E. Glass wool filtration leads to a higher percentage of spermatozoa with intact acrosomes: an ultrastructural analysis. Hum Reprod. 1998. 13:2506–2511.
Article24. Strehler E, Baccetti B, Sterzik K, Capitani S, Collodel G, De Santo M, Gambera L, Piomboni P. Detrimental effects of polyvinylpyrrolidone on the ultrastructure of spermatozoa (Notulae seminologicae 13). Hum Reprod. 1998. 13:120–123.
Article25. Thomas CA, Garner DL, Dejarnette JM, Marshall CE. Fluorometric assessments of acrosomal integrity and viability in cryopreserved bovine spermatozoa. Biol Reprod. 1997. 56:991–998.
Article26. Way AL, Henault MA, Killian GJ. Comparison of four staining methods for evaluating acrosome status and viability of ejaculated and cauda epididymal bull spermatozoa. Theriogenology. 1995. 43:1301–1316.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of percoll density gradient centrifugation in seperating human X-and Y-bearing spermatozoa
- Purification of treponema pallidum from rabbit testicular tissue
- The Effect of Sil-Select and Percoll on the Ultrastructure of Spermatozoa
- A comparative study of Sephadex, glass wool and Percoll separation techniques on sperm quality and IVF results for cryopreserved bovine semen
- Human Sperm Morphology Comparison after Pre-and Post Percoll Gradient Centrifugation