J Vet Sci.
2009 Sep;10(3):249-255. 10.4142/jvs.2009.10.3.249.
A comparative study of Sephadex, glass wool and Percoll separation techniques on sperm quality and IVF results for cryopreserved bovine semen
- Affiliations
-
- 1Laboratory of Veterinary Obstetrics and Theriogenology, College of Veterinary Medicine, Chonbuk National University, Jeonju 561-756, Korea. yjk@chonbuk.ac.kr
- KMID: 1726925
- DOI: http://doi.org/10.4142/jvs.2009.10.3.249
Abstract
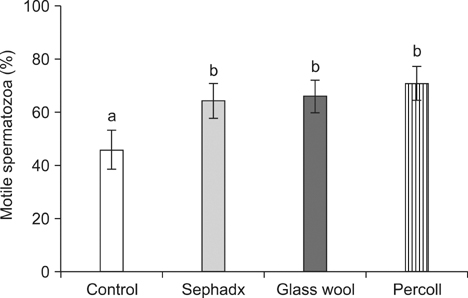
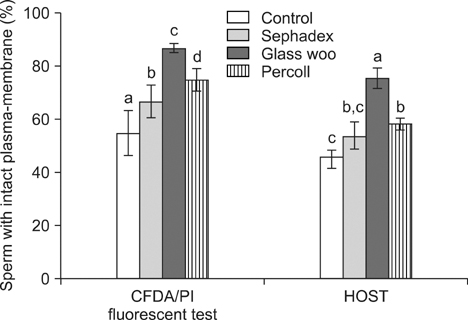
- The aim of this study was to compare the effects of spermatozoa separation techniques on sperm quality and in-vitro fertilization (IVF) results for cryopreserved bovine semen. Sephadex, glass wool and Percoll gradient separation techniques were used for sperm separation and sperm motility, morphology and membrane integrity were evaluated before and after separation. Also, cleavage and blastocyst developmental rate were investigated after IVF with sperm recovered by each separation technique. The motility of samples obtained by the three separation techniques were greater compared to the control samples (p < 0.05). The percentage of spermatozoa with intact plasma-membrane integrity, identified by 6-carboxyfluoresceindiacetate/propidium iodide fluorescent staining and the hypo-osmotic swelling test, was highest in the glass wool filtration samples (p < 0.05). The cleavage and blastocyst rate of total oocytes produced from glass wool filtration samples were also higher than the control and Sephadex filtration samples (p < 0.05), but were not significantly different from Percoll separation samples. However, a significantly greater number of cleaved embryos produced by glass wool filtration developed to blastocyst stage than those produced by Percoll separation (p < 0.05). These results indicate that spermatozoa with good quality can be achieved by these three separation techniques and can be used for bovine IVF. In particular, it suggests that glass wool filtration would be the most effective method of the three for improving sperm quality and embryo production for cryopreserved bovine spermatozoa.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Relationship between sperm quality traits and field-fertility of porcine semen
I. A. Tsakmakidis, A. G. Lymberopoulos, T. A. A. Khalifa
J Vet Sci. 2010;11(2):151-154. doi: 10.4142/jvs.2010.11.2.151.
Reference
-
1. Anzar M, Graham EF. Effect of filtration on post-thaw quality of bull semen. Theriogenology. 1995. 43:439–449.
Article2. Anzar M, Graham EF. Role of sperm motility and acrosome integrity in the filtration of bovine semen. Theriogenology. 1996. 45:513–520.
Article3. Anzar M, Graham EF, Iqbal N. Post-thaw plasma membrane integrity of bull spermatozoa separated with a Sephadex ion-exchange column. Theriogenology. 1997. 47:845–856.
Article4. Bollendorf A, Check JH, Katsoff D, Lurie D. Comparison of direct swim-up, mini-Percoll, and Sephadex G10 separation procedures. Arch Androl. 1994. 32:157–162.
Article5. Brackett BG, Oliphant G. Capacitation of rabbit spermatozoa in vitro. Biol Reprod. 1975. 12:260–274.6. Buckett WM, Luckas MJ, Aird IA, Farquharson RG, Kingsland CR, Lewis-Jones DI. The hypo-osmotic swelling test in recurrent miscarriage. Fertil Steril. 1997. 68:506–509.
Article7. Cisale HO, Fischman ML, Blasi CD, Fernandez HA, Gledhill BL. Enrichment of high-quality spermatozoa in bovine semen: relative effectiveness of three filtration matrixes. Andrologia. 2001. 33:143–150.
Article8. Coetzee K, Erasmus EL, Kruger TF, Menkveld R, Lombard CJ. Glass wool filter preparation of cryopreserved spermatozoa. Andrologia. 1994. 26:33–34.
Article9. Correa JR, Zavos PM. The hypoosmotic swelling test: Its employment as an assay to evaluate the functional integrity of the frozen-thawed bovine sperm membrane. Theriogenology. 1994. 42:351–360.
Article10. Drobnis EZ, Zhong CQ, Overstreet JW. Separation of cryopreserved human semen using Sephadex columns, washing, or Percoll gradients. J Androl. 1991. 12:201–208.11. Egozcue S, Vendrell JM, Garcia F, Veiga A, Aran B, Barri PN, Egozcue J. Increased incidence of meiotic anomalies in oligoasthenozoospermic males preselected for intracytoplasmic sperm injection. J Assist Reprod Genet. 2000. 17:307–309.12. Engel S, Weber H, Petzoldt R, Seidl B, Wiehe W, Sperl J. An improved method of sperm selection by glass wool filtration. Andrologia. 2001. 33:223–230.
Article13. Fatehi AN, Bevers MM, Schoevers E, Roelen BA, Colenbrander B, Gadella BM. DNA damage in bovine sperm does not block fertilization and early embryonic development but induces apoptosis after the first cleavages. J Androl. 2006. 27:176–188.
Article14. Gadea J, Matas C. Sperm factors related to in vitro penetration of porcine oocytes. Theriogenology. 2000. 54:1343–1357.
Article15. Goyal RL, Tuli RK, Georgie GC, Chand D. Comparison of quality and freezability of water buffalo semen after washing or Sephadex filtration. Theriogenology. 1996. 46:679–686.
Article16. Graham EF, Graham JK. The effect of whole ejaculate filtration on the morphology and the fertility of bovine semen. J Dairy Sci. 1990. 73:91–97.
Article17. Hales BF, Robaire B. Paternal exposure to drugs and environmental chemicals: effects on progeny outcome. J Androl. 2001. 22:927–936.
Article18. Hammerstedt RH, Graham JK, Nolan JP. Cryopreservation of mammalian sperm: what we ask them to survive. J Androl. 1990. 11:73–88.19. Hargreave T. Genetically determined male infertility and assisted reproduction techniques. J Endocrinol Invest. 2000. 23:697–710.
Article20. Henkel RR, Franken DR, Lombard CJ, Schill WB. Selective capacity of glass-wool filtration for the separation of human spermatozoa with condensed chromatin: a possible therapeutic modality for male-factor cases? J Assist Reprod Genet. 1994. 11:395–400.
Article21. Januskauskas A, Lukoseviciute K, Nagy S, Johannisson A, Rodriguez-Martinez H. Assessment of the efficacy of Sephadex G-15 filtration of bovine spermatozoa for cryopreservation. Theriogenology. 2005. 63:160–178.
Article22. Jaroudi KA, Carver-Ward JA, Hamilton CJ, Sieck UV, Sheth KV. Percoll semen preparation enhances human oocyte fertilization in male-factor infertility as shown by a randomized cross-over study. Hum Reprod. 1993. 8:1438–1442.
Article23. Johnson DE, Confino E, Jeyendran RS. Glass wool column filtration versus mini-Percoll gradient for processing poor quality semen samples. Fertil Steril. 1996. 66:459–462.
Article24. Kim YJ, Ji DB, Oh HG. Studies on HOS test and CTC test for viability and capacitation of frozen-thawed canine sperm (In Korean, with English abstract). Korean J Vet Clin Med. 2000. 17:431–437.25. Larson KL, Brannian JD, Timm BK, Jost LK, Evenson DP. Density gradient centrifugation and glass wool filtration of semen remove spermatozoa with damaged chromatin structure. Hum Reprod. 1999. 14:2015–2019.
Article26. Le Lannou D, Blanchard Y. Nuclear maturity and morphology of human spermatozoa selected by Percoll density gradient centrifugation or swim-up procedure. J Reprod Fertil. 1988. 84:551–556.
Article27. Lessley BA, Garner DL. Isolation of motile spermatozoa by density gradient centrifugation in Percoll. Gamete Res. 1983. 7:49–61.
Article28. Lindemann CB, Fisher M, Lipton M. A comparative study of the effects of freezing and frozen storage on intact and demembranated bull spermatozoa. Cryobiology. 1982. 19:20–28.
Article29. Mota PC, Ramalho-Santos J. Comparison between different markers for sperm quality in the cat: Diff-Quik as a simple optical technique to assess changes in the DNA of feline epididymal sperm. Theriogenology. 2006. 65:1360–1375.
Article30. Neild D, Chaves G, Flores M, Mora N, Beconi M, Aguero A. Hypoosmotic test in equine spermatozoa. Theriogenology. 1999. 51:721–727.
Article31. Oshio S. Apparent densities of spermatozoa of various mammalian species. Gamete Res. 1988. 20:159–164.
Article32. Parrish JJ, Krogenaes A, Susko-Parrish JL. Effect of bovine sperm separation by either swim-up or Percoll method on success of in vitro fertilization and early embryonic development. Theriogenology. 1995. 44:859–869.
Article33. Paulson JD, Polakoski KL. A glass wool column procedure for removing extraneous material from the human ejaculate. Fertil Steril. 1977. 28:178–181.
Article34. Rhemrev J, Jeyendran RS, Vermeiden JP, Zaneveld LJ. Human sperm selection by glass wool filtration and two-layer, discontinuous Percoll gradient centrifugation. Fertil Steril. 1989. 51:685–690.
Article35. Saeki K, Hoshi M, Leibfried-Rutledge ML, First NL. In vitro fertilization and development of bovine oocytes matured in serum-free medium. Biol Reprod. 1991. 44:256–260.
Article36. Sakkas D, Manicardi G, Bizzaro D, Bianchi PG. Possible consequences of performing intracytoplasmic sperm injection (ICSI) with sperm possessing nuclear DNA damage. Hum Fertil (Camb). 2000. 3:26–30.
Article37. Samper JC, Crabo BG. Assay of capacitated, freeze-damaged and extended stallion spermatozoa by filtration. Theriogenology. 1993. 39:1209–1220.
Article38. Sapienza F, Verheyen G, Tournaye H, Janssens R, Pletincx I, Derde M, Van Steirteghem A. An auto-controlled study in in-vitro fertilization reveals the benefit of Percoll centrifugation to swim-up in the preparation of poor-quality semen. Hum Reprod. 1993. 8:1856–1862.
Article39. Shannon P, Curson B. Toxic effect and action of dead sperm on diluted bovine semen. J Dairy Sci. 1972. 55:614–620.
Article40. Shen H, Ong C. Detection of oxidative DNA damage in human sperm and its association with sperm function and male infertility. Free Radic Biol Med. 2000. 28:529–536.
Article41. Shi Q, Martin RH. Aneuploidy in human sperm: a review of the frequency and distribution of aneuploidy, effects of donor age and lifestyle factors. Cytogenet Cell Genet. 2000. 90:219–226.
Article42. Siegel MS, Haynie LB. Effect of human sperm capacitation treatments on the penetration of freshly obtained and zona-free frozen hamster oocytes. Arch Androl. 1994. 32:5–11.
Article43. Tartagni M, Schonauer MM, Cicinelli E, Selman H, De Ziegler D, Petruzzelli F, D'Addario V. Usefulness of the hypo-osmotic swelling test in predicting pregnancy rate and outcome in couples undergoing intrauterine insemination. J Androl. 2002. 23:498–502.44. Trentalance GM, Beorlegui NB. Sperm evaluation in cryopreserved bovine semen recovered by two selection methods. Andrologia. 2002. 34:397–403.
Article45. Van den Bergh M, Revelard P, Bertrand E, Biramane J, Vanin AS, Englert Y. Glass wool column filtration, an advantageous way of preparing semen samples for intracytoplasmic sperm injection: an auto-controlled randomized study. Hum Reprod. 1997. 12:509–513.
Article46. van der Ven HH, Jeyendran RS, Al-Hasani S, Tunnerhoff A, Hoebbel K, Diedrich K, Krebs D, Perez-Pelaez M. Glass wool column filtration of human semen: relation to swim-up procedure and outcome of IVF. Hum Reprod. 1988. 3:85–88.
Article47. Zou C, Yang Z. Evaluation on sperm quality of freshly ejaculated boar semen during in vitro storage under different temperatures. Theriogenology. 2000. 53:1477–1488.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Sperm Motility, Recovery Rate, and Fertilization Rate using Three Different Sperm Preparation Methods: Swim-up, Percoll, Sil-Select
- Efficiency of Percoll Gradient, PureSperm Gradient, and Swim-up Separation Techniques in Normal Semen Samples
- Effect of Percoll Gradient Method on Sperm Number and Motility Before and after Freezing of Human Semen
- Transmission electron microscopy for characterization of acrosomal damage after Percoll gradient centrifugation of cryopreserved bovine spermatozoa
- Effects of Conversion of Infertility Treatment on Semen Quality