J Vet Sci.
2011 Sep;12(3):257-265. 10.4142/jvs.2011.12.3.257.
Influence of nitric oxide on in vitro growth, survival, steroidogenesis, and apoptosis of follicle stimulating hormone stimulated buffalo (Bubalus bubalis) preantral follicles
- Affiliations
-
- 1Reproductive Physiology Laboratory, Physiology and Climatology Division, Indian Veterinary Research Institute, Izatnagar 243122, India. gts553@gmail.com
- 2Department of Animal Science, Mahatma Jyotiba Phule Rohilkhand University, Bareilly 243006, India.
- 3Physiology and Reproduction Division, Central Avian Research Institute, Izatnagar 243122, India.
- KMID: 1067398
- DOI: http://doi.org/10.4142/jvs.2011.12.3.257
Abstract
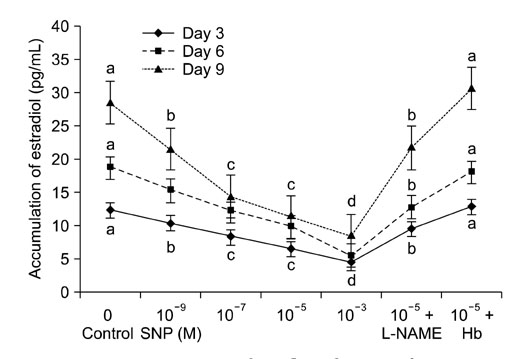
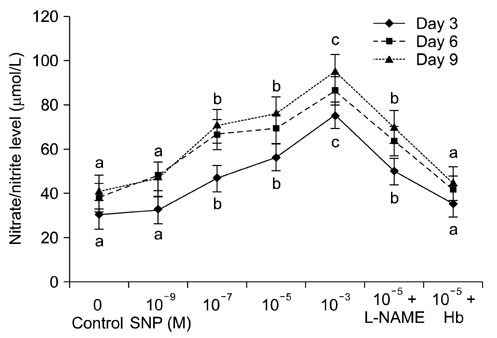
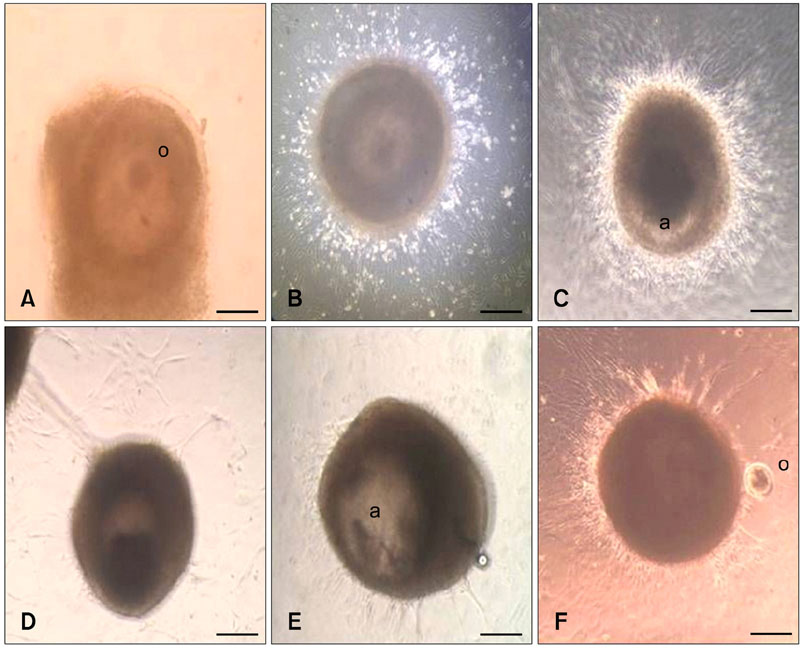
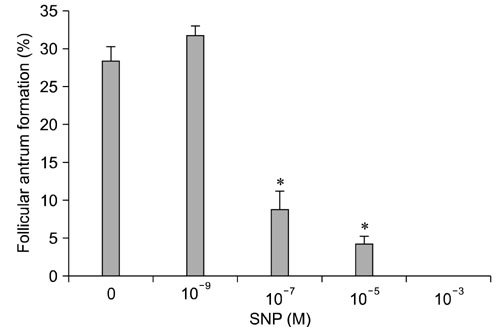
- Effect of sodium nitroprusside (SNP), a nitric oxide (NO) donor, on in vitro survival, growth, steroidogenesis, and apoptosis of buffalo preantral follicles (PFs) was investigated. PFs (200~250 microm) were isolated by micro-dissection and cultured in 0 (control), 10(-3), 10(-5), 10(-7), and 10(-9) M SNP. To examine the reversible effect of SNP, PFs were cultured with 10(-5) M SNP + 1 mM Nomega-nitro-L-arginine methyl ester (L-NAME) or 1.0 microg hemoglobin (Hb). The results showed that greater concentrations of SNP (10(-3), 10(-5), 10(-7) M) inhibited (p < 0.05) FSH-induced survival, growth, antrum formation, estradiol production, and oocyte apoptosis in a dose-dependent manner. However, a lower dose of SNP (10(-9) M) significantly stimulated (p < 0.05) the survival, growth, antrum formation, follicular oocyte maturation, and stimulated progesterone secretion compared to the control. A combination of SNP + L-NAME promoted the inhibitor effect of SNP while a SNP + Hb combination reversed this effect. Nitrate and nitrite concentrations in the culture medium increased (p < 0.05) in a dose-dependent manner according to SNP concentration in the culture medium. At higher concentrations, SNP had a cytotoxic effect leading to follicular oocyte apoptosis whereas lower concentrations have stimulatory effects. In conclusion, NO exerts a dual effect on its development of buffalo PFs depending on the concentration in the culture medium.
Keyword
MeSH Terms
-
Animals
*Apoptosis
Buffaloes/*physiology
Estradiol/biosynthesis
Female
Follicle Stimulating Hormone/metabolism
NG-Nitroarginine Methyl Ester/pharmacology
Nitrates/pharmacology
Nitric Oxide/*metabolism
Nitric Oxide Donors/pharmacology
Nitrites/pharmacology
Nitroprusside/pharmacology
Oocytes/cytology/drug effects/growth & development/metabolism
Ovarian Follicle/*cytology/drug effects/growth & development/*metabolism
Progesterone/biosynthesis
Figure
Reference
-
1. Basini G, Baratta M, Ponderato N, Bussolati S, Tamanini C. Is nitric oxide an autocrine modulator of bovine granulosa cell function? Reprod Fertil Dev. 1998. 10:471–478.
Article2. Bilodeau-Goeseels S. Effects of manipulating the nitric oxide/cyclic GMP pathway on bovine oocyte meiotic resumption in vitro. Theriogenology. 2007. 68:693–701.
Article3. Blaise GA, Gauvin D, Gangal M, Authier S. Nitric oxide, cell signaling and cell death. Toxicology. 2005. 208:177–192.
Article4. Bu S, Xia G, Tao Y, Lei L, Zhou B. Dual effects of nitric oxide on meiotic maturation of mouse cumulus cell-enclosed oocytes in vitro. Mol Cell Endocrinol. 2003. 207:21–30.
Article5. Chaube SK, Dubey PK, Mishra SK, Shrivastav TG. Verapamil reversibly inhibits spontaneous parthenogenetic activation in aged rat eggs cultured in vitro. Cloning Stem Cells. 2007. 9:608–617.
Article6. Chun SY, Eisenhauer KM, Kubo M, Hsueh AJ. Interleukin-1 beta suppresses apoptosis in rat ovarian follicles by increasing nitric oxide production. Endocrinology. 1995. 136:3120–3127.
Article7. Ellman C, Corbett JA, Misko TP, McDaniel M, Beckerman KP. Nitric oxide mediates interleukin-1-induced cellular cytotoxicity in the rat ovary. A potential role for nitric oxide in the ovulatory process. J Clin Invest. 1993. 92:3053–3056.
Article8. Faes MR, Caldas-Bussiere MC, Viana KS, Dias BL, Costa FR, Escocard RM. Nitric oxide regulates steroid synthesis by bovine antral granulosa cells in a chemically defined medium. Anim Reprod Sci. 2009. 110:222–236.
Article9. Goud PT, Goud AP, Diamond MP, Gonik B, Abu-Soud HM. Nitric oxide extends the oocyte temporal window for optimal fertilization. Free Radic Biol Med. 2008. 45:453–459.
Article10. Grasselli F, Ponderato N, Basini G, Tamanini C. Nitric oxide synthase expression and nitric oxide/cyclic GMP pathway in swine granulosa cells. Domest Anim Endocrinol. 2001. 20:241–252.
Article11. Gutierrez CG, Ralph JH, Telfer EE, Wilmut I, Webb R. Growth and antrum formation of bovine preantral follicles in long-term culture in vitro. Biol Reprod. 2000. 62:1322–1328.
Article12. Hanke CJ, Drewett JG, Myers CR, Campbell WB. Nitric oxide inhibits aldosterone synthesis by a guanylyl cyclase-independent effect. Endocrinology. 1998. 139:4053–4060.
Article13. Hsueh AJW, Billig H, Tsafriri A. Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr Rev. 1994. 15:707–724.
Article14. Jablonka-Shariff A, Olson LM. Nitric oxide is essential for optimal meiotic maturation of murine cumulus-oocyte complexes in vitro. Mol Reprod Dev. 2000. 55:412–421.
Article15. Jablonka-Shariff A, Olson LM. The role of nitric oxide in oocyte meiotic maturation and ovulation: meiotic abnormalities of endothelial nitric oxide synthase knock-out mouse oocytes. Endocrinology. 1998. 139:2944–2954.
Article16. Jablonka-Shariff A, Basuray R, Olson LM. Inhibitors of nitric oxide synthase influence oocyte maturation in rats. J Soc Gynecol Investig. 1999. 6:95–101.
Article17. Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994. 298:249–258.
Article18. Lamas S, Marsden PA, Li GK, Tempst P, Michel T. Endothelial nitric oxide synthase: molecular cloning and characterization of a distinct constitutive enzyme isoform. Proc Natl Acad Sci USA. 1992. 89:6348–6352.
Article19. Li J, Billiar TR. The role of nitric oxide in apoptosis. Semin Perinatol. 2000. 24:46–50.
Article20. Li HY, Chang SP, Yuan CC, Chao HT, Ng HT, Sung YJ. Nitric oxide induces extensive apoptosis in endometrial epithelial cells in the presence of progesterone: involvement of mitogen-activated protein kinase pathways. Mol Hum Reprod. 2001. 7:755–763.
Article21. Mao J, Wu G, Smith MF, McCauley TC, Cantley TC, Prather RS, Didion BA, Day BN. Effects of culture medium, serum type, and various concentrations of follicle-stimulating hormone on porcine preantral follicular development and antrum formation in vitro. Biol Reprod. 2002. 67:1197–1203.
Article22. Masuda M, Kubota T, Kamada S, Aso T. Nitric oxide inhibits steroidogenesis in cultured porcine granulosa cells. Mol Hum Reprod. 1997. 3:285–292.
Article23. Matta SGC, Caldas-Bussiere MC, Viana KS, Faes MR, Paes de Carvalho CS, Dias BL, Quirino CR. Effect of inhibition of synthesis of inducible nitric oxide synthase-derived nitric oxide by aminoguanidine on the in vitro maturation of oocyte-cumulus complexes of cattle. Anim Reprod Sci. 2009. 111:189–201.
Article24. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991. 43:109–142.25. Morris SM Jr, Billiar TR. New insights into the regulation of inducible nitric oxide synthesis. Am J Physiol. 1994. 266:E829–E839.
Article26. Olson LM, Jablonka-Shariff A, Beltsos AN. Adashi EY, editor. Ovarian nitric oxide: a modulator of ovulation and oocyte maturation. Ovulation: Evolving Scientific and Clinical Concepts. 2000. New York: Springer;243–254.
Article27. Orsi NM. Embryotoxicity of the nitric oxide donor sodium nitroprusside in preimplantation bovine embryons in vitro. Anim Reprod Sci. 2006. 91:225–236.
Article28. Pires PRL, Santos NP, Adona PR, Natori MM, Schwarz KRL, de Bem THC, Leal CLV. Endothelial and inducible nitric oxide synthases in oocytes of cattle. Anim Reprod Sci. 2009. 116:233–243.
Article29. Sarti P, Lendaro E, Ippoliti R, Bellelli A, Benedetti PA, Brunori M. Modulation of mitochondrial respiration by nitric oxide: investigation by single cell fluorescence microscopy. FASEB J. 1999. 13:191–197.
Article30. Sastry KVH, Moudgal RP, Mohan J, Tyagi JS, Rao GS. Spectrophotometric determination of serum nitrite and nitrate by copper-cadmium alloy. Anal Biochem. 2002. 306:79–82.
Article31. Schwarz KRL, Pires PRL, Adona PR, Câmara de Bem TH, Leal CLV. Influence of nitric oxide during maturation on bovine oocyte meiosis and embryo development in vitro. Reprod Fertil Dev. 2008. 20:529–536.
Article32. Sengoku K, Takuma N, Horikawa M, Tsuchiya K, Komori H, Sharifa D, Tamate K, Ishikawa M. Requirement of nitric oxide for murine oocyte maturation, embryo development, and trophoblast outgrowth in vitro. Mol Reprod Dev. 2001. 58:262–268.
Article33. Sharma GT, Dubey PK, Meur SK. Survival and developmental competence of buffalo preantral follicles using three dimensional collagen gel culture system. Anim Reprod Sci. 2009. 114:115–124.
Article34. Sharma GT, Dubey PK, Meur SK. Effect of different mechanical isolation techniques on developmental competence and survival of buffalo ovarian preantral follicles. Livest Sci. 2009. 123:300–305.
Article35. Sharma GT, Loganathasamy K. Effect of meiotic stages during in vitro maturation on the survival of vitrified-warmed buffalo oocytes. Vet Res Commun. 2007. 31:881–893.
Article36. Snyder GD, Holmes RW, Bates JN, Van Voorhis BJ. Nitric oxide inhibits aromatase activity: mechanisms of action. J Steroid Biochem Mol Biol. 1996. 58:63–69.
Article37. Tamanini C, Basini G, Grasselli F, Tirelli M. Nitric oxide and the ovary. J Anim Sci. 2003. 81:E Suppl 2. E1–E7.38. Tao Y, Fu Z, Zhang MJ, Xia GL, Yang J, Xie HR. Immunohistochemical localization of inducible and endothelial nitric oxide synthase in porcine ovaries and effects of NO on antrum formation and oocyte meiotic maturation. Mol Cell Endocrinol. 2004. 222:93–103.
Article39. Van Voorhis BJ, Dunn MS, Snyder GD, Weiner CP. Nitric oxide: an autocrine regulator of human granulosa-luteal cell steroidogenesis. Endocrinology. 1994. 135:1799–1806.
Article40. Viana KS, Caldas-Bussiere MC, Matta SGC, Faes MR, Paes de Carvalho CS, Quirino CR. Effect of sodium nitroprusside, a nitric oxide donor, on the in vitro maturation of bovine oocytes. Anim Reprod Sci. 2007. 102:217–227.
Article41. Zhang J, Wang S, Kern S, Cui X, Danner RL. Nitric oxide down-regulates polo-like kinase 1 through a proximal promoter cell cycle gene homology region. J Biol Chem. 2007. 282:1003–1009.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In vitro Follicular Growth and Ovulation of Mouse Preantral Follicles Cryopreserved by Vitrification
- Control Mechanisms of Ovarian Follicle Development by Follicle Stimulating Hormone and Pituitary Adenylate Cyclase-activating Polypeptide
- In vitro growth of mouse preantral follicles: effect of animal age and stem cell factor/insulin-like growth factor supplementation
- Impact of vitamin D3 supplementation on the in vitro growth of mouse preantral follicles
- Survival of isolated human preantral follicles after vitrification: Analyses of morphology and Fas ligand and caspase-3 mRNA expression