J Vet Sci.
2006 Jun;7(2):181-187. 10.4142/jvs.2006.7.2.181.
Random amplified polymorphic DNA (RAPD) analysis of Mycobacterium tuberculosis strains in India
- Affiliations
-
- 1Division of Bacteriology and Mycology, Indian Veterinary Research Institute, Izatnagar-243122, U.P., India. rishendra_verma@yahoo.com
- 2Division Veterinary Biotechnology, Indian Veterinary Research Institute, Izatnagar-243122, U.P., India.
- KMID: 1059206
- DOI: http://doi.org/10.4142/jvs.2006.7.2.181
Abstract
-
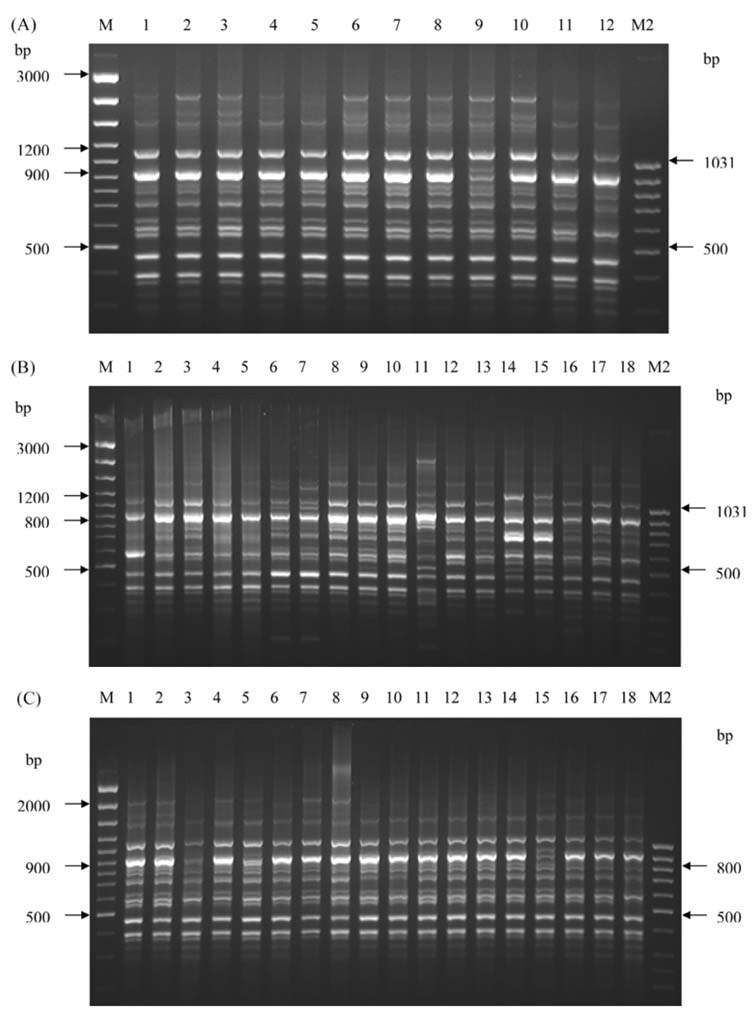
The usefulness of random amplification of polymorphic
DNA (RAPD) analysis for typing Indian strains of M.
tuberculosis was investigated. M. tuberculosis H37Rv, M.
tuberculosis DT and 42 clinical isolates of M. tuberculosis
were subjected to RAPD-PCR using 7 random decamer
primers. All 7 primers were found to be differentiated and
produced specific RAPD profiles. The polymorphic amplicons
served as RAPD markers for M. tuberculosis. The
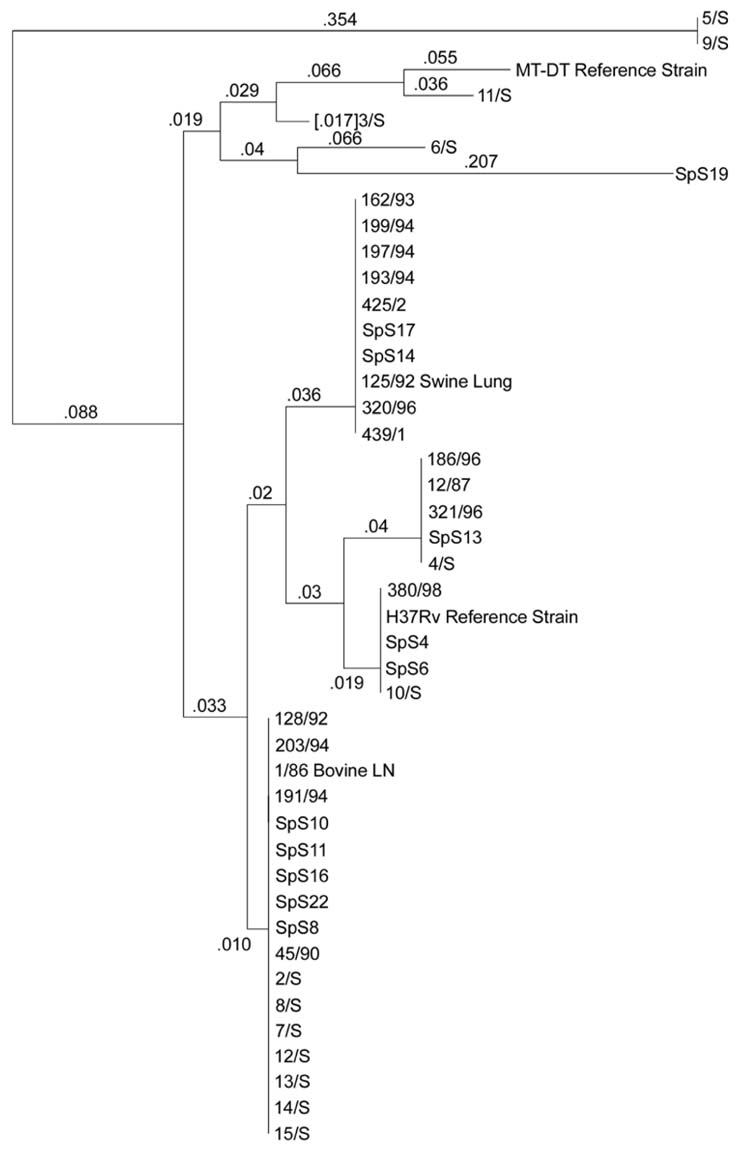
dendrograms, obtained by different primers, showed the
discriminatory ability of the primers. RAPD analysis
provided a rapid and easy means of identifying polymorphism
in M. tuberculosis isolates, and it was found to be a
valuable alternative epidemiological tool. In addition, the
results
of the present study showed heterogeneity in the M. tuberculosis strains in the population studied.
Keyword
MeSH Terms
-
Animals
Cattle
Cattle Diseases/microbiology
DNA, Bacterial/*analysis/genetics
Gene Expression Profiling
Humans
India/epidemiology
Lung/microbiology
Mycobacterium tuberculosis/*genetics/*isolation&purification
Phylogeny
*Random Amplified Polymorphic DNA Technique
Sputum/microbiology
Swine
Swine Diseases/microbiology
Tuberculosis, Pulmonary/*epidemiology/*microbiology/veterinary
Variation (Genetics)
Figure
Reference
-
1. Blazquez J, Espinosa de Los Monteros LE, Samper S, Martin C, Guerrero A, Cobo J, van Embden J, Baquero F, Gomez-Mampaso E. Genetic characterization of multidrug-resistant Mycobacterium bovis strains from a hospital outbreak involving human immunodeficiency virus-positive patients. J Clin Microbiol. 1997. 35:1390–1393.
Article2. Bloom BR, Murray CJ. Tuberculosis: Commentary on a reemergent killer. Science. 1992. 257:1055–1064.
Article3. Bowditch BM, Albright DG, Williams JG, Braun MJ. Use of randomly amplified polymorphic DNA markers in comparative genome studies. Methods Enzymol. 1993. 224:294–309.4. Caetano-Anolles G. Amplifying DNA with arbitrary oligonucleotide primers. PCR Methods Appl. 1991. 3:85–94.
Article5. Chansiripornchai N, Ramasoota P, Sasipreeyajan J, Svenson SB. Differentiation of avian pathogenic E. coli (APEC) strains by random amplified polymorphic DNA (RAPD) analysis. Vet Microbiol. 2001. 80:75–83.
Article6. Cosivi O, Grange JM, Daborn CJ, Raviglione MC, Fujikura T, Cousins D, Robinson RA, Huchzermeyer HF, de Kantor I, Meslin FX. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg Infect Dis. 1998. 4:59–70.
Article7. Dziva F, Christensen H, Olsen JE, Mohan K. Random amplification of polymorphic DNA and phenotypic typing of Zimbabwean isolates of Pasteurella multocida. Vet Microbiol. 2001. 82:361–372.
Article8. Harn HJ, Shen KL, Ho LI, Yu KW, Liu GC, Yueh KC, Lee JH. Evidence of transmission of Mycobacterium tuberculosis by random amplified polymorphic DNA (RAPD) fingerprinting in Taipei City, Taiwan. J Clin Pathol. 1997. 50:505–508.
Article9. Hermans K, De Herdt P, Baele M, Devriese LA, Haesebrouck F. Sequence analysis or a RAPD band differentiating high and low virulence Staphylococcus aureus strains from rabbits. Vet Microbiol. 2001. 82:61–67.
Article10. Jones WD Jr, Kubica GP. Fluorescent antibody technique with mycobacteria. III. Investigation of five serologically homogenous groups of mycobacteria. Zentralbl Bakteriol Orig. 1968. 207:58–62.11. Jones WD Jr. Geographic distribution of phage types among cultures of Mycobacterium tuberculosis. Am Rev Respir Dis. 1990. 142:1000–1003.
Article12. Katoch VM, Singh D, Chauhan DS, Sharma VD, Singh HB, Das R, Srivastava K. Mahajan RC, Therwath A, editors. Newer DNA fingerprinting techniques for tuberculosis-relevance in control. Multi-drug resistance in emerging and re-emerging diseases. 2000. New Delhi: Indian Science Academy & Narosa Publishing House;87–96.13. Linton CJ, Jalal H, Leeming JP, Millar MR. Rapid discrimination of Mycobacterium tuberculosis strains by random amplified polymorphic DNA analysis. J Clin Microbiol. 1994. 32:2169–2174.
Article14. Menard C, Brousseau R, Mouton C. Application of polymerase chain reaction with arbitrary primer (AP-PCR) to strain identification of Porphyromonas (Bacteroides) gingivalis. FEMS Microbiol Lett. 1992. 74:163–168.15. Michalak K, Austin C, Diesel S, Bacon JM, Zimmerman P, Maslows JN. Mycobacterium tuberculosis infection as a zoonotic disease; transmission between humans and elephants. Emerg Infect Dis. 1998. 4:283–287.
Article16. Perumaalla VS, Adams LG, Payeur J, Baca D, Ficht TA. Molecular fingerprinting confirms extensive cow-to-cow intra-herd transmission of a single Mycobacterium bovis strain. Vet Microbiol. 1999. 70:269–276.
Article17. Richner SM, Meiring J, Kirby R. A study of the genetic diversity of Mycobacterium tuberculosis isolated from patients in the eastern province of South Africa using random amplified polymorphic DNA profiling. Electrophoresis. 1997. 18:1570–1576.
Article18. Snider DE Jr, Jones WD, Good RC. The usefulness of phage typing Mycobacterium tuberculosis isolates. Am Rev Respir Dis. 1984. 130:1095–1099.19. Rynyon EH, Karlson AG, Kubica GP, Wayne LG. Lennette EH, Balows A, Hausler Jr WJ, Truant JP, editors. Mycobacterium. Manual of Clinical Microbiology. 1980. 3rd ed. Washington DC: American Society for Microbiology;150–179.20. Singh HB, Chauhan DS, Singh D, Das R, Srivastava K, Yadav VS, Kumar A, Katoch VM, Sharma VD. Rapid discrimination of Indian isolates of M. tuberculosis by random amplified polymorphic DNA (RAPD) analysis - A preliminary report. Indian J Med Microbiol. 2002. 20:69–71.21. Singh SK, Verma R, Shah DH. Molecular fingerprinting of clinical isolates of Mycobacterium bovis and Mycobacterium tuberculosis from India by restriction fragment length polymorphism (RFSP). J Vet Sci. 2004. 5:331–335.
Article22. Tazi L, El Baghdadi J, Lesjean S, Locht C, Supply P, Tibayrenc M, Banuls AL. Genetic Diversity and Population Structure of Mycobacterium tuberculosis in Casablanca, a Moroccan City with high incidence of tuberculosis. J Clin Microbiol. 2004. 42:461–466.
Article23. van Soolingen D, de Haas PEW, Kremer K. Restriction Fragment Length Polymorphism (RFLP) typing of mycobacteria. 1999. Bilthoven, Netherlands: National Institute of Public Health and Enivronment.24. van Soolingen D, Hoogenboezem T, de Haas PE, Hermans PW, Koedam MA, Teppema KS, Brennan PJ, Besra GS, Portaels F, Top J, Schouls LM, van Embden JD. A novel pathogenic taxon of the Mycobacterium tuberculosis complex, canetti: Characterization of an exceptional isolate from Africa. Int J Syst Bacteriol. 1997. 47:1236–1245.
Article25. Verma R, Singh HB, Sharma VD, Katoch VM. Molecular characterization of Indian Mycobacterium bovis isolates by Random Amplified Polymorphic DNA (RAPD) Analysis -A Preliminary Report. Indian J Vet Res. 2002. 11:39–41.26. Verma R, Srivastava SK. Mycobacterium isolated from man and animals; twelve year record. Indian J Anim Sci. 2001. 71:129–132.27. Welsh J, McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990. 18:7213–7218.
Article28. Williams JG, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV. DNA polymorphism amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990. 18:6531–6535.
Article29. World health organization (WHO). Global tuberculosis control. WHO report 2001. 2001. Geneva: WHO.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecular Epidemiological Characteristics of Drug-resistant Mycobacterium tuberculosis by Random Amplified Polymorphic DNA Genotyping
- Identification of Trichophyton tonsurans by Random Amplified Polymorphic DNA
- Identification of Dermatophytes by Mycological Tests and Random Amplified Polymorphic DNA analysis
- Genotypic Identification of Fusarium subglutinans, F. proliferatum and F. verticillioides Strains Isolated from Maize in Austria
- Plasmid DNA and Random Amplified Polymorphic DNA (RAPD) Analyses of Vancomycin-Resistant Enterococcus faecium Isolates