Current Status of External Quality Assessment of Syphilis Test in Korea
- Affiliations
-
- 1Department of Laboratory Medicine, Konkuk University College of Medicine, Seoul, Korea.
- 2Department of Laboratory Medicine, Chung-Ang University Hospital, Seoul, Korea.
- 3Department of Laboratory Medicine, Dongguk University College of Medicine, Goyang, Korea.
- 4Department of Laboratory Medicine, Chung-Ang University College of Medicine, Seoul, Korea. chayoung@cau.ac.kr
- KMID: 854869
- DOI: http://doi.org/10.3343/kjlm.2008.28.3.207
Abstract
- BACKGROUND
Current status of external quality assessment (EQA) of laboratory tests for syphilis in Korea was analyzed to find out the problems that should be improved in the future.
METHODS
Based on the data from the external quality assessment program performed twice a year by the Immunoserology Subcommittee of the Korean Association of Quality Assurance for Clinical Laboratory from the year 2004 to 2006, discordance rates were analyzed according to the test method and commercial kit used.
RESULTS
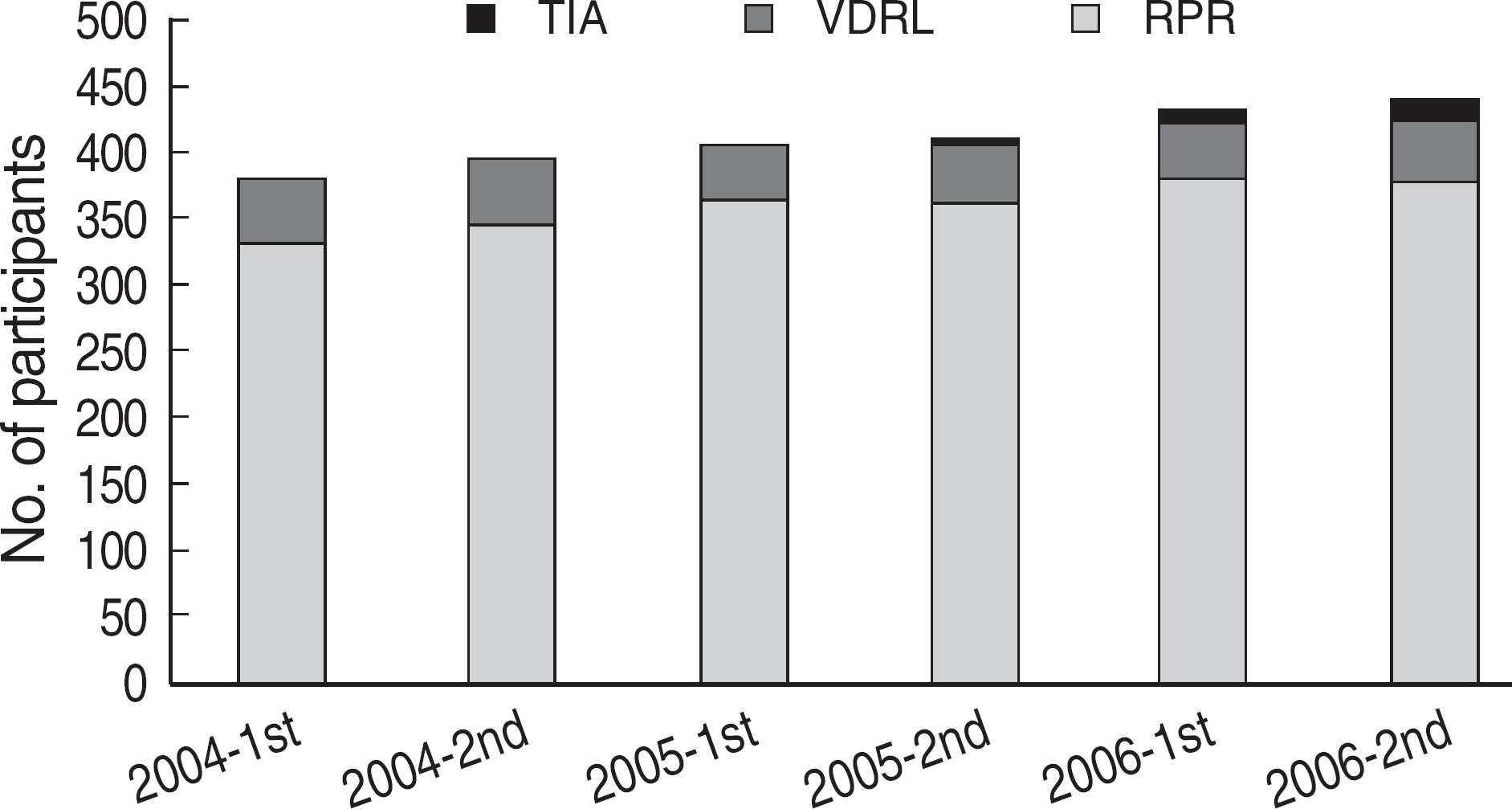
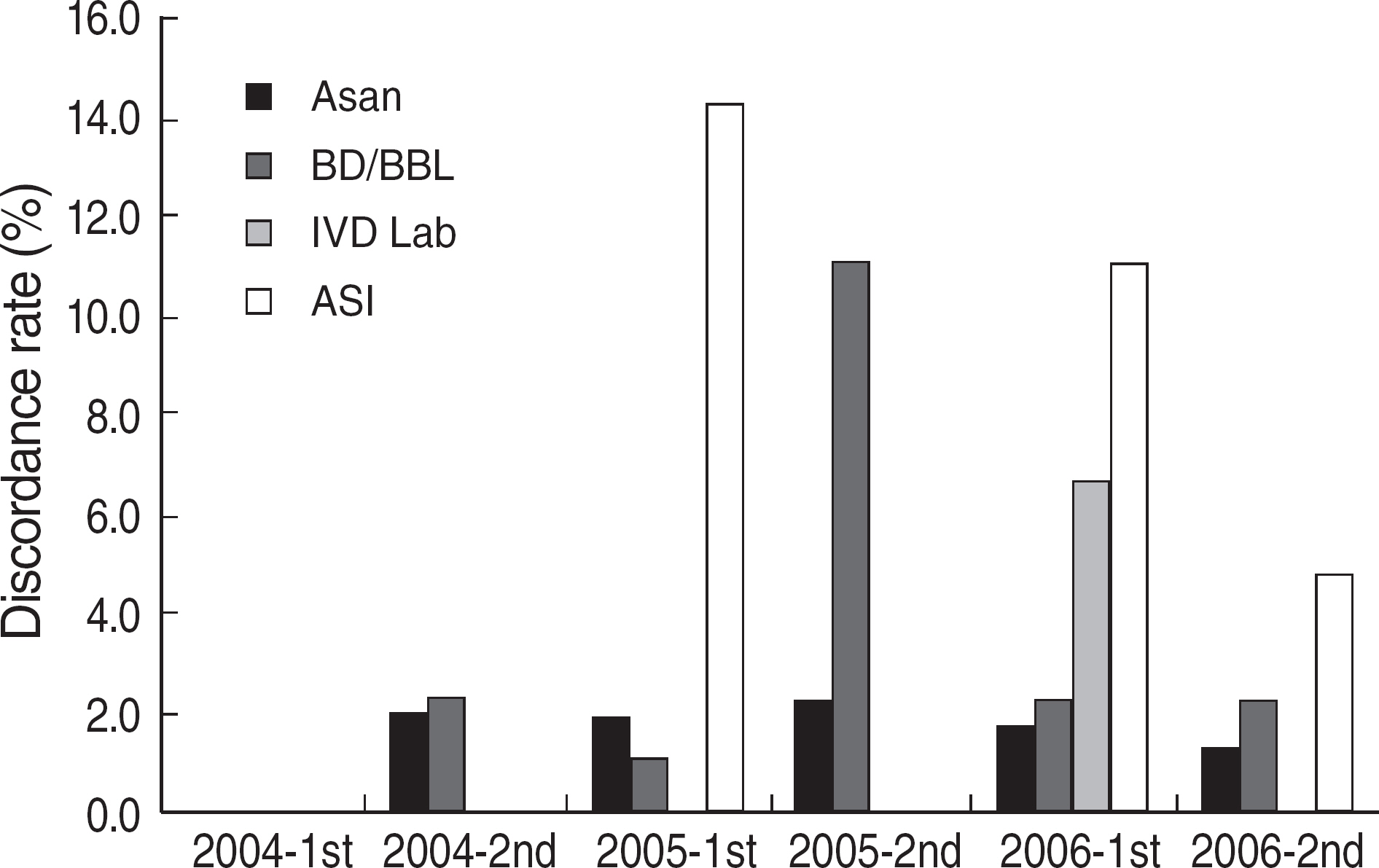
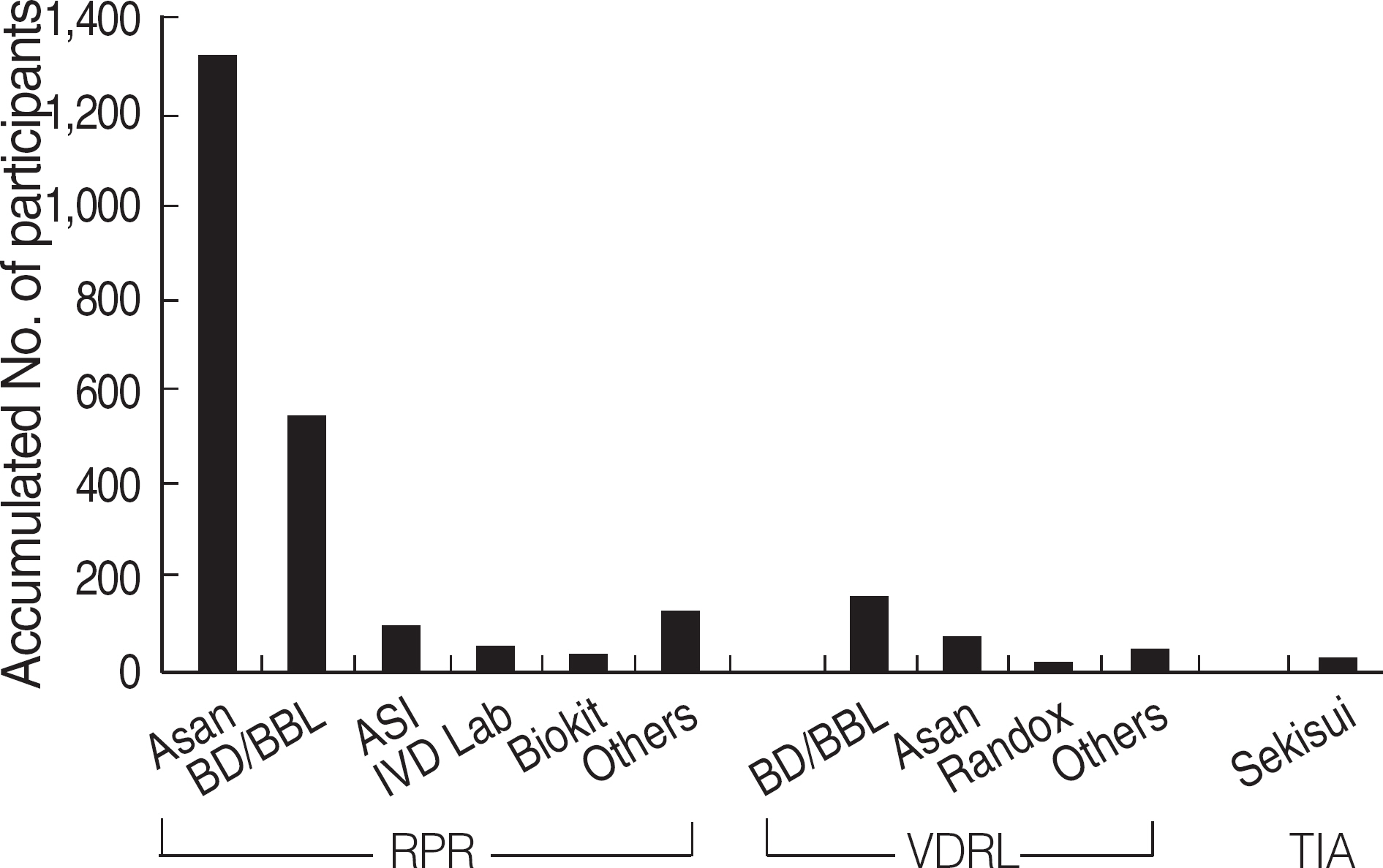
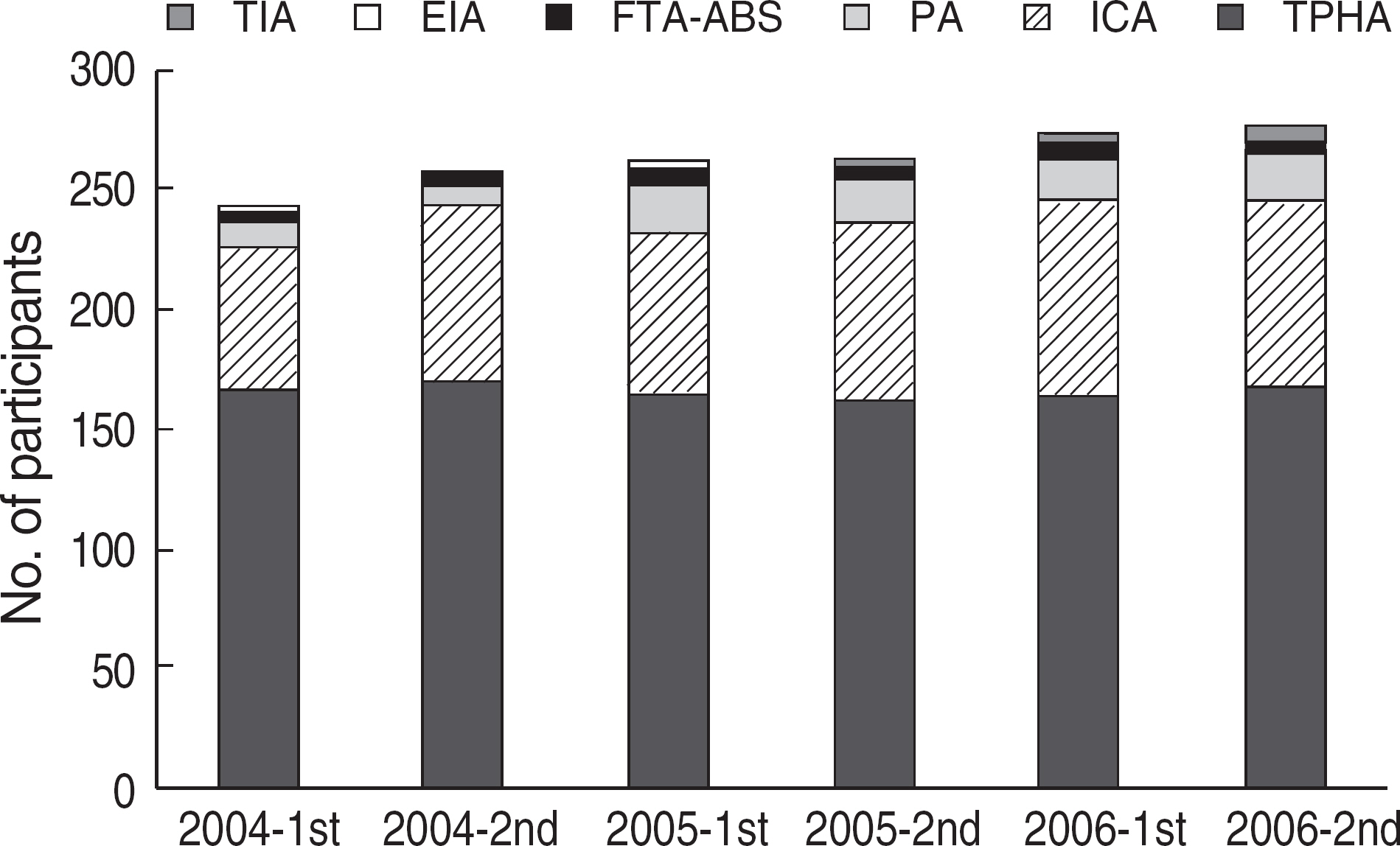
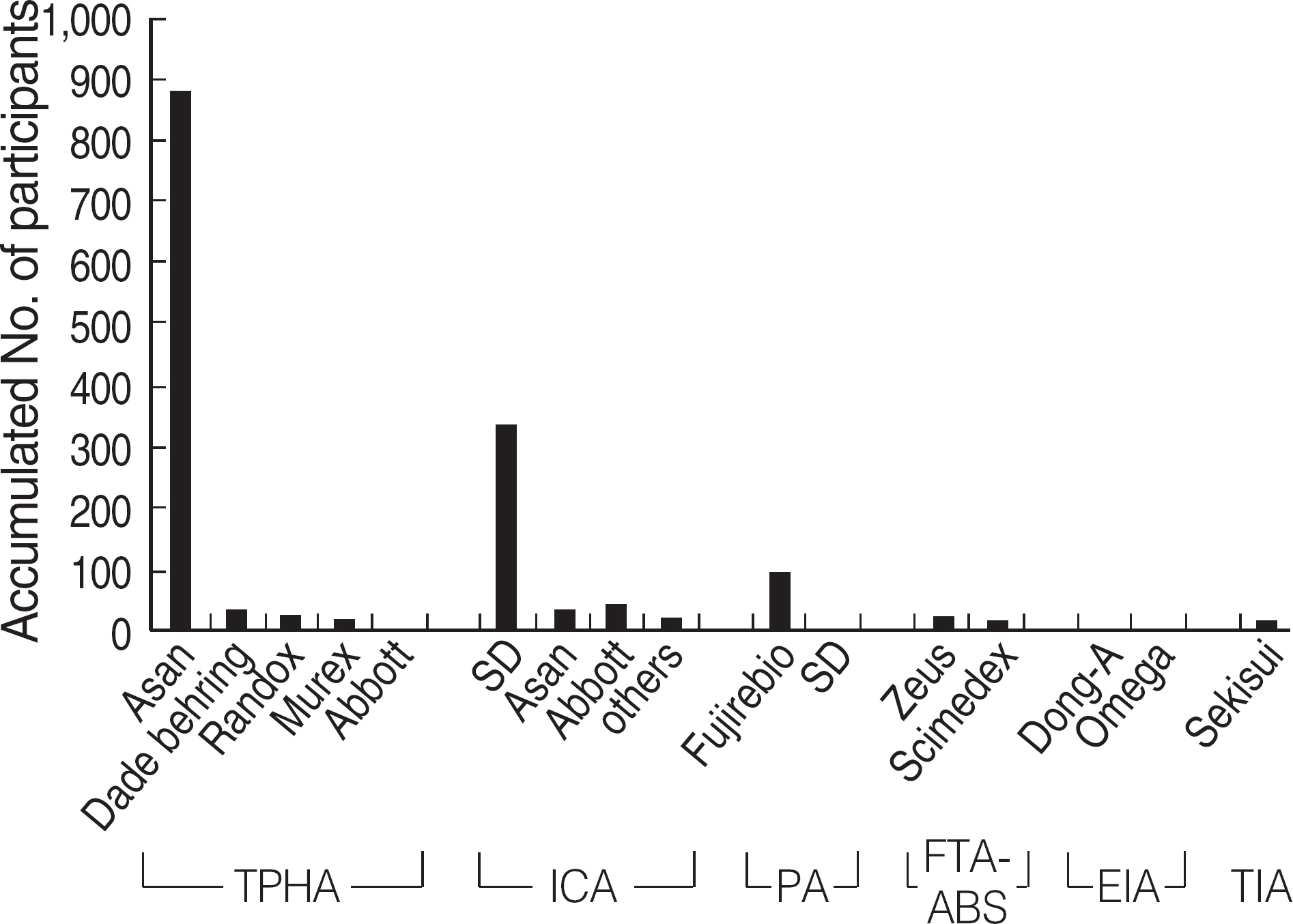
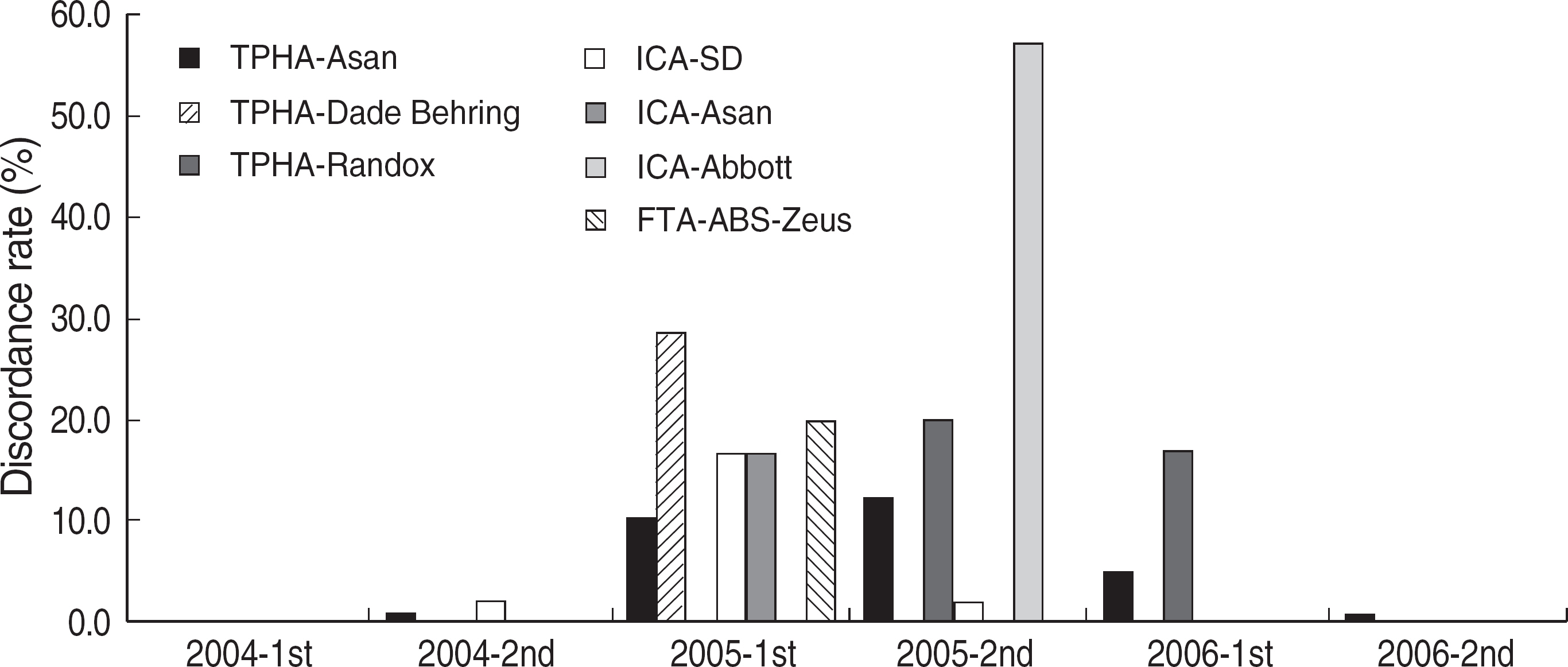
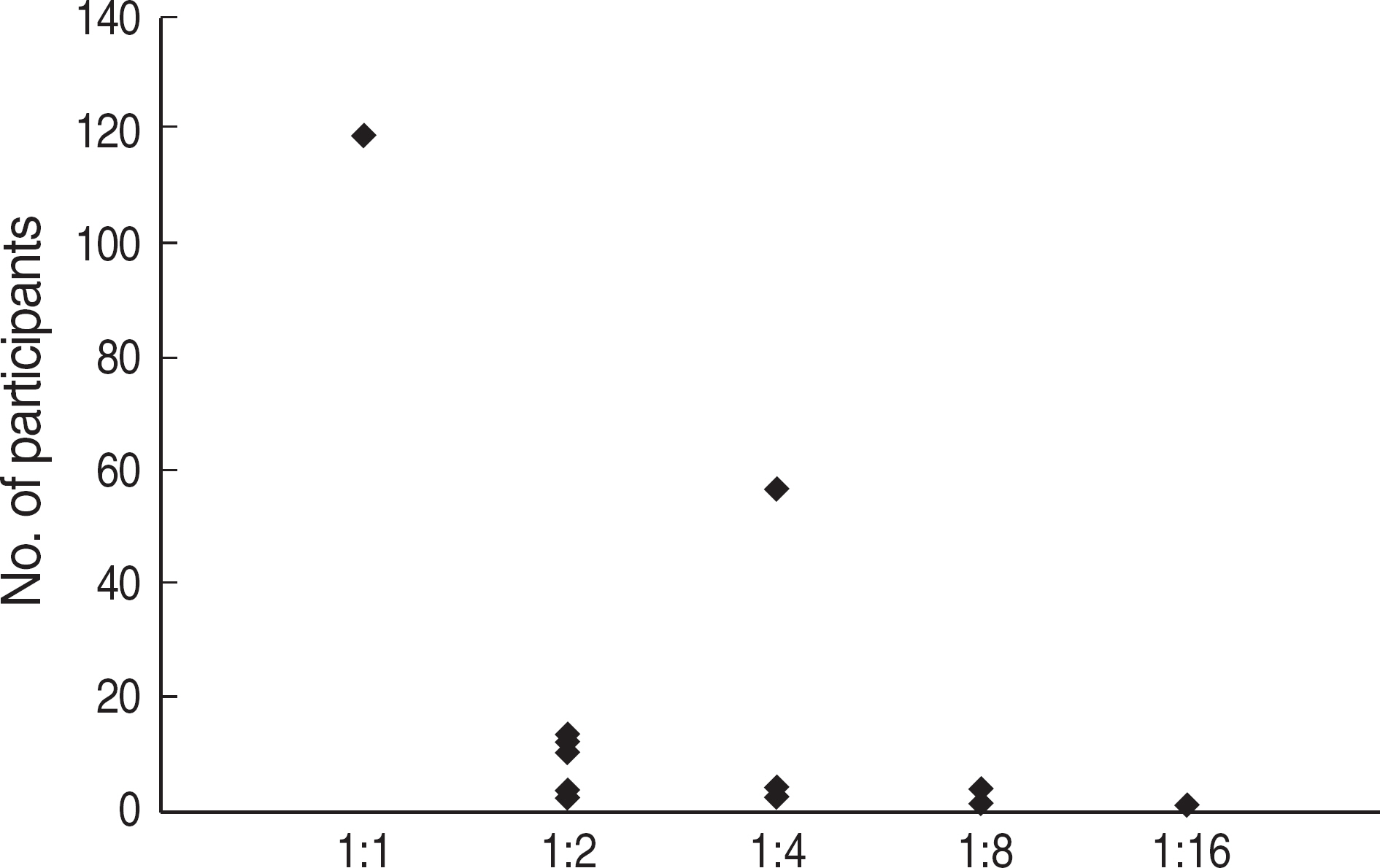
Among the laboratories participating in the EQA program for syphilis test, about 90% of them used non-treponemal tests and about 55% treponemal tests. The non-treponemal tests included RPR (rapid plasma reagin) and VDRL tests used in 88% (363/412) and 11% (45/412), respectively, of the laboratories. The discordance rates were 2.2% for RPR test and 3.6% for VDRL. For the treponemal tests, Treponema pallidum hemagglutination assay (TPHA) was used in 60-76% and Immunochromatography assay (ICA) in about 30% of the laboratories in 2006. A high discordance rate of over 10% was reported in both TPHA and in ICA methods, possibly due to a low titer (1:1 in VDRL) of EQA samples in 2005. Analysis of the accumulated data from year 2004 to 2006 showed that the discordance rates of TPHA, ICA, and FTA-ABS were 4.6%, 3.7%, and 2.7%, respectively.
CONCLUSIONS
For syphilis tests, RPR test, TPHA, and ICA are mainly used in Korea. A high discordance rate is still reported in TPHA and ICA, especially when testing samples with a low titer. Further analysis of data and education of laboratory personnel are needed for the improvement of the EQA program.
MeSH Terms
Figure
Cited by 5 articles
-
Establishment and Multicenter Evaluation of a National Reference Panel for Syphilis Antibodies in Korea
Hee Jin Huh, Seok Lae Chae, Deok-Ja Oh, Quehn Park, Chae Seung Lim, Tae Hyun Um, Yun Mi Park, Young Joo Cha
Lab Med Online. 2014;4(1):36-42. doi: 10.3343/lmo.2014.4.1.36.Evaluation of Centaur Syphilis, Immulite Syphilis, and Mediace TPLA for Detecting Treponemal Antibodies
Dong Hee Seo, Dong Hee Whang, Shin Young Joo, Hyen Hee Choi
Lab Med Online. 2015;5(2):77-83. doi: 10.3343/lmo.2015.5.2.77.Comparison of Automated Treponemal and Nontreponemal Test Algorithms as First-Line Syphilis Screening Assays
Hee Jin Huh, Jae-Woo Chung, Seong Yeon Park, Seok Lae Chae
Ann Lab Med. 2016;36(1):23-27. doi: 10.3343/alm.2016.36.1.23.Comparison of Auto RPR Plus and Auto TPIM Plus with Mediace RPR and Abbott Syphilis TP for Serologic Diagnosis of Syphilis
Hyun-Jeong Kim, Eun-Hee Nah, Seon Cho, So-Young Jeong
Lab Med Online. 2018;8(3):87-93. doi: 10.3343/lmo.2018.8.3.87.Evaluation of Automated Architect Syphilis TP as a Diagnostic Laboratory Screening Test for Syphilis
Jeeyong Kim, Woo-Hyeun Kim, Chihyun Cho, Juyeon Kim, Ga-Yeong Kim, Myung-Hyun Nam, Jang Su Kim, Sook Young Bae, Yunjung Cho
Korean J Lab Med. 2008;28(6):475-482. doi: 10.3343/kjlm.2008.28.6.475.
Reference
-
1.Peterman TA., Heffelfinger JD., Swint EB., Groseclose SL. The changing epidemiology of syphilis. Sex Transm Dis. 2005. 32(S):S4–10.
Article2.Larsen SA., Steiner BM., Rudolph AH. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev. 1995. 8:1–21.
Article3.Hsu WS., Kao JT., Ho SW. Quality assurance in clinical laboratories in Taiwan. J Formos Med Assoc. 2000. 99:235–42.4.Neimeister RP., Teschemacher R., Yankevitch IJ., Cocklin J. Proficiency testing, trouble shooting and quality control for the RPR test. Am J Med Technol. 1975. 41:13–7.5.Snell JJ., De Mello JV., Gardner PS. The United Kingdom national microbiological quality assessment scheme. J Clin Pathol. 1982. 35:82–93.
Article6.Taylor RN., Fulford KM., Przybyszewski VA., Pope V. Center for Disease Control Diagnostic Immunology Proficiency Testing Program results for 1977. J Clin Microbiol. 1978. 8:388–95.
Article7.Taylor RN., Fulford KM. Assessment of laboratory improvement by the Center for Disease Control Diagnostic Immunology Proficiency Testing Program. J Clin Microbiol. 1981. 13:356–68.
Article8.Muller I., Brade V., Hagedorn HJ., Straube E., Schorner C., Frosch M, et al. Is serological testing a reliable tool in laboratory diagnosis of syphilis? Meta-analysis of eight external quality control surveys performed by the German infection serology proficiency testing program. J Clin Microbiol. 2006. 44:1335–41.
Article9.Cha YJ., Kwon SY., Kum DG., Kim SW., Kim TY., Kim JR, et al. Annual report on external quality assessment in immunoserology in Korea (2003). J Lab Med Qual Assur. 2004. 26:47–69. (차영주, 권소영, 금동길, 김성원, 김신규, 김재룡등. 면역혈청검사신빙도조사결과보고 (2003). 임 상검사와정도관리 2004;26: 47-69.).10.Cha YJ., Kwon SY., Kum DG., Kim SW., Kim TY., Kim JR, et al. Annual report on external quality assessment in immunoserology in Korea (2004). J Lab Med Qual Assur. 2005. 27:37–57. (차영주, 권소영, 금동길, 김성원, 김신규, 김재룡 등. 면역혈청검사 신빙도조사 결과보고 (2004). 임 상검사와정도관리 2005;27: 37-57.).11.Cha YJ., Kwon SY., Kim TY., Kim JR., Kim HS., Park MH, et al. Annual report on external quality assessment in Immunoserology in Korea (2005). J Lab Med Qual Assur. 2006. 28:41–61. (차영주, 권소영, 김신규, 김재룡, 김현숙, 박명희 등. 면역혈청검사 신빙도조사 결과보고 (2005). 임상검사와정도관리 2006;28: 41-61.).12.Cha YJ., Kwon SY., Kim TY., Kim JR., Kim HS., Park MH, et al. Annual report on external quality assessment in immunoserology in Korea (2006). J Lab Med Qual Assur. 2007. 29:45–64. (차영주, 권소영, 김신규, 김재룡, 김현숙, 박명희등. 면역혈청검사신빙도조사결과보고(2006). 임상검사와정도관리 2007;29: 45-64.).13.College of American Pathologists. Surveys 2005 G-A Syphilis Serology Participant Summary, CAP. 2005.14.College of American Pathologists. Surveys 2005 G-B Syphilis Serology Participant Summary, CAP. 2005.15.College of American Pathologists. Surveys 2005 G-C Syphilis Serology Participant Summary, CAP. 2005.16.College of American Pathologists. Surveys 2006 G-A Syphilis Serology Participant Summary, CAP. 2006.17.College of American Pathologists. Surveys 2006 G-B Syphilis Serology Participant Summary, CAP. 2006.18.College of American Pathologists. Surveys 2006 G-C Syphilis Serology Participant Summary, CAP. 2006.19.Castro AR., Kikkert SE., Fears MB., Pope V. Defibrination of blood plasma for use in serological tests for syphilis. Clin Diagn Lab Immunol. 2002. 9:1376–8.
Article20.Cole M., Dean L., Perry KR., Parry JV. Five syphilis agglutination assays, MHRA Report number 04007. London: Medicines and Healthcare products Regulatory Agency;2004.21.Mabey D., Peeling RW., Ballard R., Benzaken AS., Galban E., Changalucha J, et al. Prospective, multi-centre clinic-based evaluation of four rapid diagnostic tests for syphilis. Sex Transm Infect. 2006. 82(S):v13–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Report of the Korean Association of External Quality Assessment Service on Serological Tests for Syphilis (2020–2021)
- Acquired Secondary Syphilis in Early Childhood
- Current Status of Serum Allergen Tests in Korea
- Report of the Korean Association of External Quality Assessment Service on Serologic Tests for Syphilis (2018–2019)
- Annual Report on the External Quality Assessment Scheme of Viral Markers and Serological Tests for Syphilis in Korea (2014)