Nontuberculous Mycobacterial Pulmonary Diseases in Immunocompetent Patients
- Affiliations
-
- 1Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. ojkwon@smc.samsung.co.kr
- 2Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 754056
- DOI: http://doi.org/10.3348/kjr.2002.3.3.145
Abstract
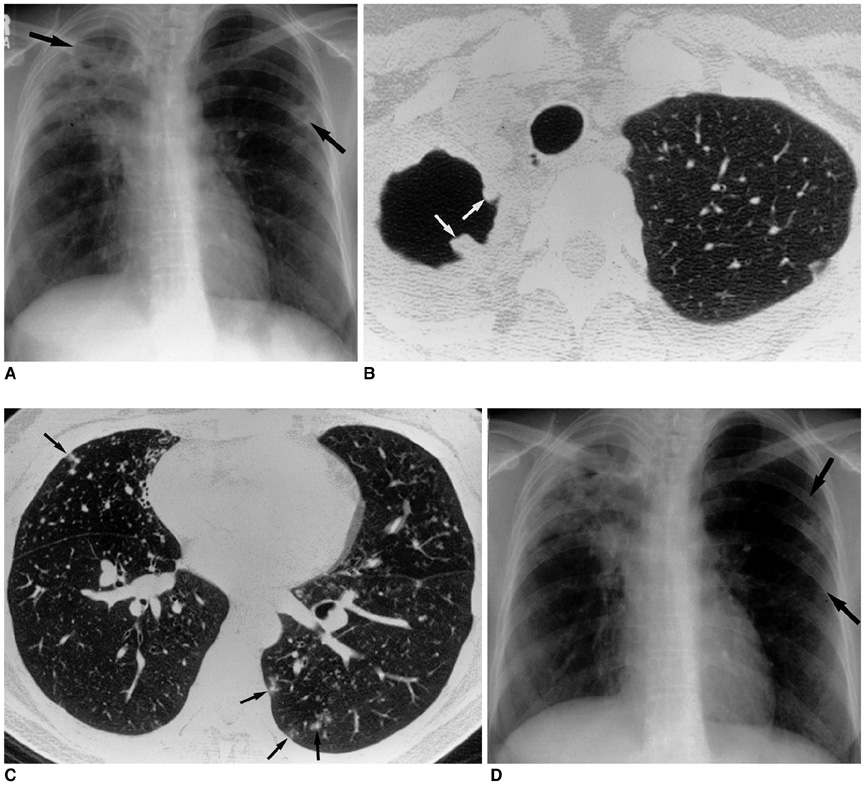
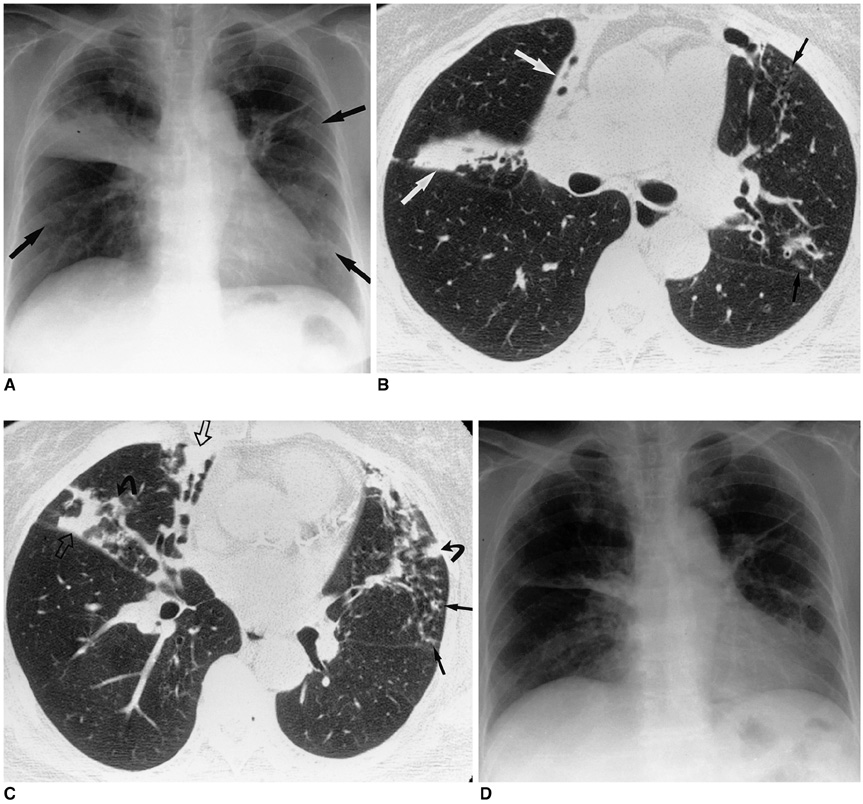
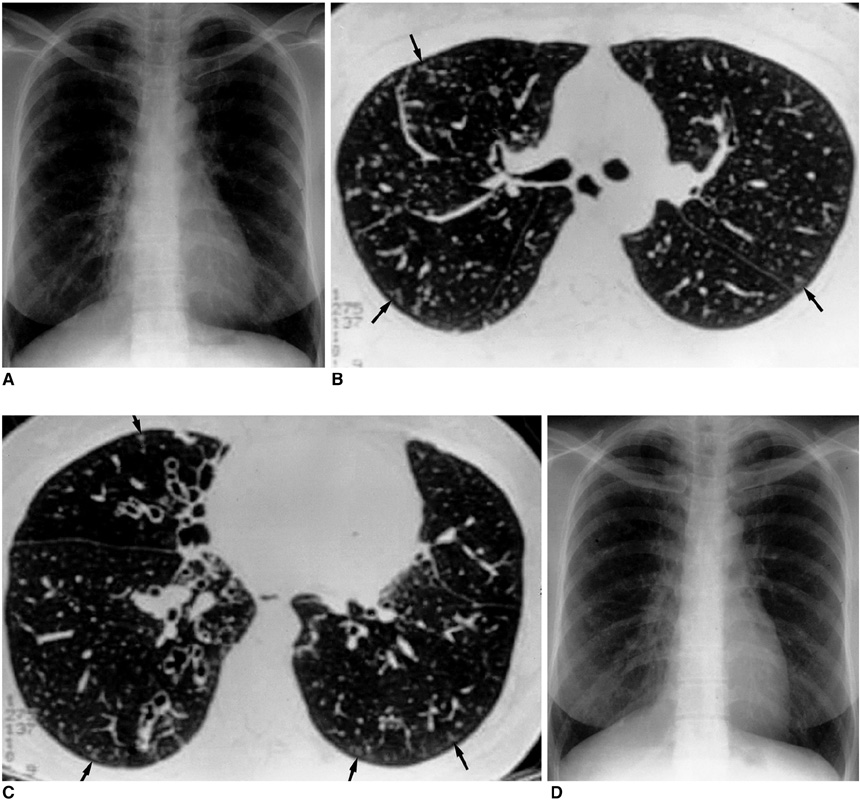
- Nontuberculous mycobacterial (NTM) infections are an increasingly recognized cause of chronic lung disease in immunocompetent adults, and the M. avium complex, M. kansasii, and a rapidly growing mycobacteria such as M. abscessus, M. fortuitum, and M. chelonae account for most of the pathogens involved. Because the clinical features of NTM disease are not distinguishable from those of tuberculosis, and NTM are ubiquitous in the environment, diagnosis requires that the bacilli are isolated and identified. NTM diseases have been difficult to treat, though since the introduction of new macrolides, the outcome for patients with some NTM diseases has improved significantly. For correct diagnosis and the successful treatment of NTM pulmonary disease, a knowledge of the full spectrum of clinical and radiological findings is important.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Diagnosis and Treatment of Nontuberculous Mycobacterial Pulmonary Diseases: A Korean Perspective
Won-Jung Koh, O Jung Kwon, Kyung Soo Lee
J Korean Med Sci. 2005;20(6):913-925. doi: 10.3346/jkms.2005.20.6.913.Evaluation of the Broth Microdilution Method Using 2,3-Diphenyl-5-thienyl-(2)-tetrazolium Chloride for Rapidly Growing Mycobacteria Susceptibility Testing
Sun Min Lee, Jeong man Kim, Joseph Jeong, Young Kil Park, Gill-Han Bai, Eun Yup Lee, Min Ki Lee, Chulhun L. Chang
J Korean Med Sci. 2007;22(5):784-790. doi: 10.3346/jkms.2007.22.5.784.Cytokine Profiles of Macrophages by
Mycobacterium abscessus Conditional Morphotype Variants and Comparison of Their Extracellular-Protein Expressions
Kil-Soo Lee, Hosung Sohn, Seung-Sub Lee, Byung Soo Lee, Hwa-Jung Kim, Sung Jae Shin
J Bacteriol Virol. 2008;38(3):109-118. doi: 10.4167/jbv.2008.38.3.109.
Reference
-
1. American Thoracic Society. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am J Respir Crit Care Med. 1997. 156:S1–S25.2. British Thoracic Society. Management of opportunistic mycobacterial infections: Joint Tuberculosis Committee guidelines, 1999. Thorax. 2000. 55:210–218.3. Wolinsky E. Nontuberculous mycobacteria and associated diseases. Am Rev Respir Dis. 1979. 119:107–159.4. Tsukamura M, Kita N, Shimoide H, Arakawa H, Kuze A. Studies on the epidemiology of nontuberculous mycobacteriosis in Japan. Am Rev Respir Dis. 1988. 137:1280–1284.5. O'Brien RJ, Geiter LJ, Snider DE Jr. The epidemiology of nontuberculous mycobacterial diseases in the United States: results from a notional survey. Am Rev Respir Dis. 1987. 135:1007–1014.6. Jin BW, Saito H, Yoshii Z. Environmental mycobacteria in Korea: I. Distribution of the organisms. Microbiol Immunol. 1984. 28:667–677.7. Ministry of Health & Social Affairs. Korean National Tuberculosis Association. The 2nd report on the 6th tuberculosis prevalence survey in Korea. 1990. Seoul: 1–11. (in Korean).8. Kim SJ, Hong YP, Kim SC, Bai GH, Jin BW, Park CD. A case of pulmonary disease due to Mycobacterium avium-intracellulare complex. Tuberc Lung Dis. 1981. 28:121–124. (in Korean).9. Kim SJ, Hong YP, Bai GH, Kim SC, Jin BW. Nontuberculous pulmonary infection in two patients with Mycobacterium avium-intracellulare complex and a patient with M. fortuitum. J Kor Soc Microbiol. 1982. 17:87–93. (in Korean).10. Kim HJ, Oh SH, Lee WY, Kim SJ. Report of a case of pulmonary mycobacteriosis caused by Mycobacterium chelonei subsp. abscessus. Tuberc Lung Dis. 1985. 32:54–57. (in Korean).11. Yim JJ, Oh MD, Yoo CG, et al. A case of Mycobacterium abscessus pneumonia in a patient with systemic lupus erythematosus. Tuber Lung Dis. 1999. 46:96–102. (in Korean).12. Kim EK, Shim TS, Lim CM, et al. Clinical features of Mycobacterium chelonae pulmonary infection. Tuberc Lung Dis. 2001. 51:Suppl 2. 85. (abstract). (in Korean).13. Kim DK, Kim BJ, Kook YH, et al. Pulmonary infection with Mycobacterium celatum in immunocompetent host: first case report in Korea. Tuberc Lung Dis. 1999. 47:697–703. (in Korean).14. Bai GH, Park KS, Kim SJ. Clinically isolated mycobacteria other than Mycobacterium tuberculosis from 1980 to 1990 in Korea. J Korean Soc Microbiol. 1993. 28:1–5. (in Korean).15. Lew WJ, Ahn DI, Yoon YJ, et al. Clinical experience of mycobacterial diseases other than tuberculosis. Tuberc Lung Dis. 1992. 39:425–432. (in Korean).16. Korean Academy of Tuberculosis and Respiratory Diseases. National survey of mycobacterial diseases other than tuberculosis in Korea. Tuberc Lung Dis. 1995. 42:277–294. (in Korean).17. Yoon CJ, Goo JM, Seo JB, Kim SH, Im JG. CT findings of mycobacterial infection other than tuberculosis: comparison with tuberculosis. J Korean Radiol Soc. 2000. 42:487–492. (in Korean).18. Park SY, Park JH, Jegal YJ, et al. A case of idiopathic CD4+ T-lymphocytopenia with disseminated Mycobacterium kansasii infection and pulmonary alveolar proteinosis. Tuberc Respir Dis. 2000. 48:377–382. (in Korean).19. The Mycobacteriosis Research Group of the Japanese National Chest Hospitals. Rapid increase of the incidence of lung disease due to Mycobacterium kansasii in Japan. Chest. 1983. 83:890–892.20. Christensen EE, Dietz GW, Ahn CH, et al. Initial roentgenographic manifestations of pulmonary Mycobacterium tuberculosis, M. kansasii, and M. intracellularis infections. Chest. 1981. 80:132–136.21. Ahn CH, McLarty JW, Ahn SS, Ahn SI, Hurst GA. Diagnostic criteria for pulmonary disease caused by Mycobacterium kansasii and Mycobacterium intracellulare. Am Rev Respir Dis. 1982. 125:388–391.22. Miller WT Jr. Spectrum of pulmonary nontuberculous mycobacterial infection. Radiology. 1994. 191:343–350.23. Erasmus JJ, McAdams HP, Farrell MA, Patz EF Jr. Pulmonary nontuberculous mycobacterial infection: radiologic manifestations. RadioGraphics. 1999. 19:1487–1505.24. Woodring JH, Vandiviere HM. Pulmonary disease caused by nontuberculous mycobacteria. J Thorac Imaging. 1990. 5:64–76.25. Hartman TE, Swensen SJ, Williams DE. Mycobacterium avium-intracellulare complex: evaluation with CT. Radiology. 1993. 187:23–26.26. Swensen SJ, Hartman TE, Williams DE. Computed tomographic diagnosis of Mycobacterium avium-intracellulare complex in patients with bronchiectasis. Chest. 1994. 105:49–52.27. Lynch DA, Simone PM, Fox MA, Bucher BL, Heinig MJ. CT features of pulmonary Mycobacterium avium complex infection. J Comput Assist Tomogr. 1995. 19:353–360.28. Primack SL, Logan PM, Hartman TE, Lee KS, Müller NL. Pulmonary tuberculosis and Mycobacterium avium-intracellulare: a comparison of CT findings. Radiology. 1995. 194:413–417.29. Corbett EL, Blumberg L, Churchyard GJ, et al. Nontuberculous mycobacteria: defining disease in a prospective cohort of South African miners. Am J Respir Crit Care Med. 1999. 160:15–21.30. World Health Organization. Global Tuberculosis Programme. Publication reference WHO/GTB/96.210. Treatment of Tuberculosis: Guidelines for National Programmes. 1997. 2nd ed. Geneva, Switzerland: World Health Organization.31. Woodring JH, Vandiviere HM, Melvin IG, Dillon ML. Roentgenographic features of pulmonary disease caused by atypical mycobacteria. South Med J. 1987. 80:1488–1497.32. Christensen EE, Dietz GW, Ahn CH, et al. Pulmonary manifestations of Mycobacterium intracellulare. AJR. 1979. 133:59–66.33. Prince DS, Peterson DD, Steiner RM, et al. Infection with Mycobacterium avium complex in patients without predisposing conditions. N Engl J Med. 1989. 321:863–868.34. Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern. Chest. 1992. 101:1605–1609.35. Tanaka E, Amitani R, Niimi A, Suzuki K, Murayama T. Yield of computed tomography and bronchoscopy for the diagnosis of Mycobacterium avium complex pulmonary disease. Am J Respir Crit Care Med. 1997. 155:2041–2046.36. Fujita J, Ohtsuki Y, Suemitsu I, et al. Pathological and radiological changes in resected lung specimens in Mycobacterium avium-intracellulare complex disease. Eur Respir J. 1999. 13:535–540.37. Huang JH, Kao PN, Adi V, Ruoss SJ. Mycobacterium avium-intracellulare pulmonary infection in HIV-negative patients without preexisting lung disease: diagnostic and management limitations. Chest. 1999. 115:1033–1040.38. Moore EH. Atypical mycobacterial infection in the lung: CT appearance. Radiology. 1993. 187:777–782.39. Wallace RJ Jr, Zhang Y, Brown BA, et al. Polyclonal Mycobacterium avium complex infections in patients with nodular bronchiectasis. Am J Respir Crit Care Med. 1998. 158:1235–1244.40. American Thoracic Society. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am Rev Respir Dis. 1990. 142:940–953.41. BritishThoracic Society. First randomised trial of treatments for pulmonary disease caused by M. avium-intracellulare, M. malmoense, and M. xenopi in HIV negative patients: rifampicin, ethambutol and isoniazid versus rifampicin and ethambutol. Thorax. 2001. 56:167–172.42. Wallace RJ Jr, Brown BA, Griffith DE, Girard WM, Murphy DT. Clarithromycin regimens for pulmonary Mycobacterium avium complex: The first 50 patients. Am J Respir Crit Care Med. 1996. 153:1766–1772.43. Dautzenberg B, Piperno D, Diot P, Truffot-Pernot C, Chauvin JP. Clarithromycin in the treatment of Mycobacterium avium lung infections in patients without AIDS. Chest. 1995. 107:1035–1040.44. Tanaka E, Kimoto T, Tsuyuguchi K, et al. Effect of clarithromycin regimen for Mycobacterium avium complex pulmonary disease. Am J Respir Crit Care Med. 1999. 160:866–872.45. Johanson WG Jr, Nicholson DP. Pulmonary disease due to Mycobacterium kansasii: an analysis of some factors affecting prognosis. Am Rev Respir Dis. 1969. 99:73–85.46. Christensen EE, Dietz GW, Ahn CH, Chapman JS, Murry RC, Hurst GA. Radiographic manifestations of pulmonary Mycobacterium kansasii infections. AJR. 1978. 131:985–993.47. Evans AJ, Crisp AJ, Hubbard RB, Colville A, Evans SA, Johnston ID. Pulmonary Mycobacterium kansasii infection: comparison of radiological appearances with pulmonary tuberculosis. Thorax. 1996. 51:1243–1247.48. Ahn CH, Lowell JR, Ahn SS, Ahn SI, Hurst GA. Short-course chemotherapy for pulmonary disease caused by Mycobacterium kansasii. Am Rev Respir Dis. 1983. 128:1048–1050.49. Griffith DE, Girard WM, Wallace RJ Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria: an analysis of 154 patients. Am Rev Respir Dis. 1993. 147:1271–1278.50. Wallace RJ Jr, Swenson JM, Silcox VA, Good RC, Tschen JA, Stone MS. Spectrum of disease due to rapidly growing mycobacteria. Rev Infect Dis. 1983. 5:657–679.51. Wallace RJ Jr. Diagnostic and therapeutic consideration in patients with pulmonary disease due to the rapidly growing mycobacteria. Semin Respir Infect. 1986. 1:230–233.52. Hazelton TR, Newell JD Jr, Cook JL, Huitt GA, Lynch DA. CT findings in 14 patients with Mycobacterium chelonae pulmonary infection. AJR. 2000. 175:413–416.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pulmonary infection with Mycobacterium celatum in immunocompetent host: The first case report in Korea
- A Case Report of Three Patients with Nontuberculous Mycobacterial Pulmonary Disease Caused by Mycobacterium kansasii
- Chronic Large Non Healing Ulcer: Non-Tuberculous Mycobacterial Infection of the Laryngopharynx
- Nontuberculous Mycobacterial Pulmonary Infection in Immunocompetent Patients
- Radiologic Diagnosis of Nontuberculous Mycobacterial Pulmonary Disease