CT Diagnosis of Fitz-Hugh and Curtis Syndrome: Value of the Arterial Phase Scan
- Affiliations
-
- 1Department of Diagnostic Radiology, Yonsei University College of Medicine, Seoul, Korea. kimnex@yumc.yonsei.ac.kr
- 2Brain Korea 21 Project for Medical Science, Korea.
- 3Institute of Gastroenterology, Korea.
- KMID: 753864
- DOI: http://doi.org/10.3348/kjr.2007.8.1.40
Abstract
OBJECTIVE
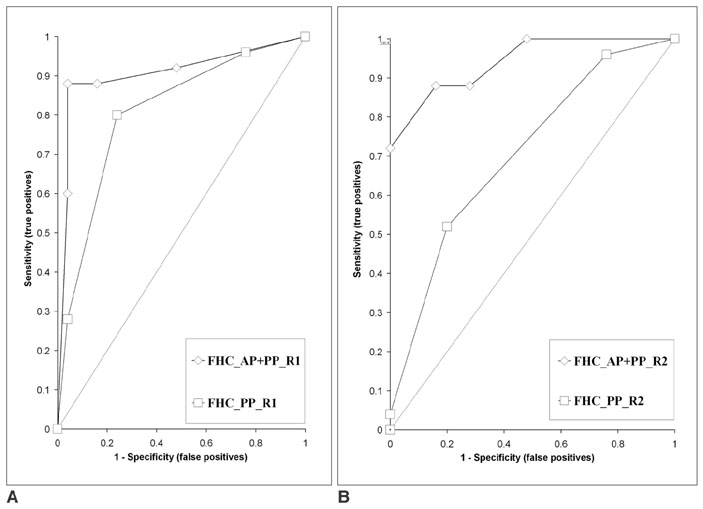
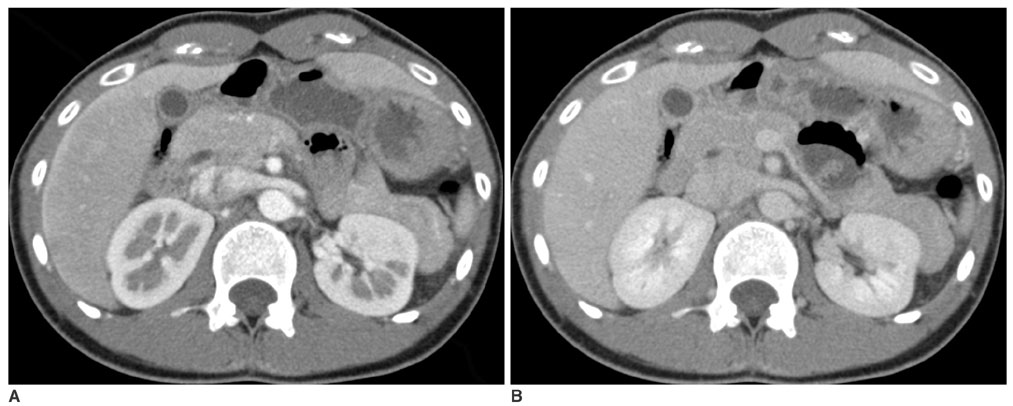
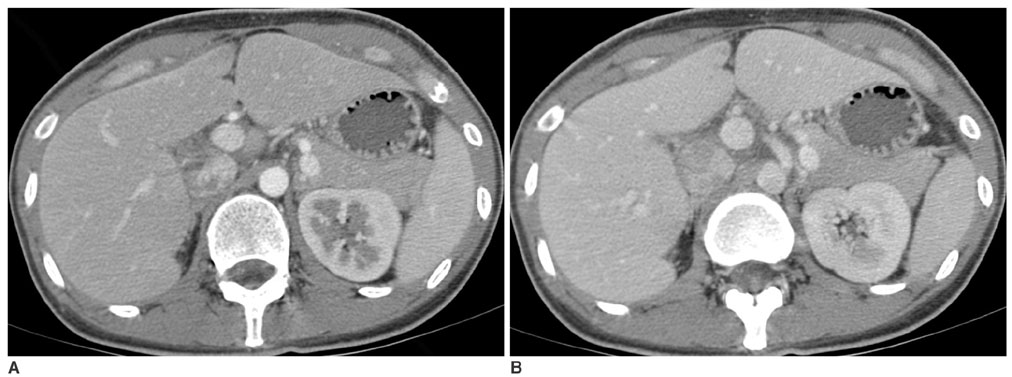
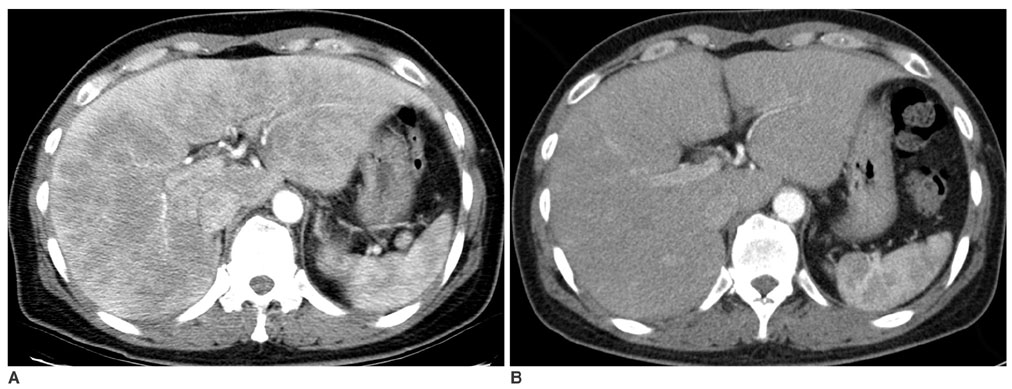
We wanted to evaluate the role of the arterial phase (AP) together with the portal venous phase (PP) scans in the diagnosis of Fitz-Hugh-Curtis syndrome (FHCS) with using computed tomography (CT). MATERIALS AND METHODS: Twenty-five patients with FHCS and 25 women presenting with non-specifically diagnosed acute abdominal pain and who underwent biphasic CT examinations were evaluated. The AP scan included the upper abdomen, and the PP scan included the whole abdomen. Two radiologists blindly and retrospectively reviewed the PP scans first and then they reviewed the AP plus PP scans. The diagnostic accuracy of FHCS on each image set was compared for each reader by analyzing the area under the receiver operating characteristic curve (Az). Weighted kappa (wk) statistics were used to measure the interobserver agreement for the presence of CT signs of the pelvic inflammatory disease (PID) on the PP images and FHCS as the diagnosis based on the increased perihepatic enhancement on both sets of images. RESULTS: The individual diagnostic accuracy of FHCS was higher on the biphasic images (Az = 0.905 and 0.942 for reader 1 and 2, respectively) than on the PP images alone (Az = 0.806 and 0.706, respectively). The interobserver agreement for the presence of PID on the PP images was moderate (wk = 0.530). The interobserver agreement for FHCS as the diagnosis was moderate on only the PP images (wk = 0.413), but it was substantial on the biphasic images (wk = 0.719). CONCLUSION: Inclusion of the AP scan is helpful to depict the increased perihepatic enhancement, and it improves the diagnostic accuracy of FHCS on CT.
MeSH Terms
Figure
Cited by 4 articles
-
Clinical Features of Fitz-Hugh-Curtis Syndrome in the Emergency Department
Je Sung You, Min Joung Kim, Hyun Soo Chung, Yong Eun Chung, Incheol Park, Sung Phil Chung, Seungho Kim, Hahn Shick Lee
Yonsei Med J. 2012;53(4):753-758. doi: 10.3349/ymj.2012.53.4.753.Frequency of N. gonorrheaee, C. trachomatis, U. urealyticum and M. hominis in Pelvic Inflammatory Disease and Fitz-Hugh-Curtis Syndrome
Gyoung Hoon Lee, Hye Ji Kim, Chul Hi Park, Yoon Jung Chun, Hyun Jung Choi, Han Na Lee, Sook Cho
Infect Chemother. 2012;44(5):362-366. doi: 10.3947/ic.2012.44.5.362.A Case of Fitz-Hugh-Curtis syndrome in a male patient
Si Hyeong Lee, Ju Il Yang, Jung Sik Choi
Kosin Med J. 2018;33(2):223-227. doi: 10.7180/kmj.2018.33.2.223.A Case of Fitz-Hugh-Curtis Syndrome in a Male
Hyun Choul Baek, Young Seok Bae, Kwang Jae Lee, Dong Hyun Kim, Sang Hoon Bae, Dong Wan Kim, Jung Bin Yoon, Chul Soo Song
Korean J Gastroenterol. 2010;55(3):203-207. doi: 10.4166/kjg.2010.55.3.203.
Reference
-
1. Peter NG, Clark LR, Jaeger JR. Fitz-Hugh-Curtis syndrome: a diagnosis to consider in women with right upper quadrant pain. Cleve Clin J Med. 2004. 71:233–239.2. Counselman FL. An unusual presentation of Fitz-Hugh-Curtis syndrome. J Emerg Med. 1994. 12:167–170.3. Paavonen J, Saikku P, von Knorring J, Aho K, Wang SP. Association of infection with Chlamydia trachomatis with Fitz-Hugh-Curtis syndrome. J Infect Dis. 1981. 144:176.4. Litt IF, Cohen MI. Perihepatitis associated with salpingitis in adolescents. JAMA. 1978. 240:1253–1254.5. Dinerman LM, Elfenbein DS, Cumming WA. Clinical Fitz-Hugh-Curtis syndrome in an adolescent. Ultrasonographic findings. Clin Pediatr (Phila). 1990. 29:532–535.6. Banerjee B, Rennison A, Boyes BE. Sonographic features in a case of Fitz-Hugh-Curtis syndrome masquerading as malignancy. Br J Radiol. 1992. 65:342–344.7. Schoenfeld A, Fisch B, Cohen M, Vardy M, Ovadia J. Ultrasound findings in perihepatitis associated with pelvic inflammatory disease. J Clin Ultrasound. 1992. 20:339–342.8. van Dongen PW. Diagnosis of Fitz-Hugh-Curtis syndrome by ultrasound. Eur J Obstet Gynecol Reprod Biol. 1993. 50:159–162.9. Piscaglia F, Vidili G, Ugolini G, Ramini R, Montroni I, De Iaco P, et al. Fitz-Hugh-Curtis-syndrome mimicking acute cholecystitis: value of new ultrasound findings in the differential diagnosis. Ultraschall Med. 2005. 26:227–230.10. Nishie A, Yoshimitsu K, Irie H, Yoshitake T, Aibe H, Tajima T, et al. Fitz-Hugh-Curtis syndrome. Radiologic manifestation. J Comput Assist Tomogr. 2003. 27:786–791.11. Tsubuku M, Hayashi S, Terahara A, Furukawa T, Ohmura G. Fitz-Hugh-Curtis syndrome: linear contrast enhancement of the surface of the liver on CT. J Comput Assist Tomogr. 2002. 26:456–458.12. Pickhardt PJ, Fleishman MJ, Fisher AJ. Fitz-Hugh-Curtis syndrome: multidetector CT findings of transient hepatic attenuation difference and gallbladder wall thickening. AJR Am J Roentgenol. 2003. 180:1605–1606.13. Mesurolle B, Mignon F, Gagnon JH. Fitz-Hugh-Curtis syndrome caused by Chlamydia trachomatis: atypical CT findings. AJR Am J Roentgenol. 2004. 182:822–824. author reply 824.14. Chevalier N, De Tayrac R, Dagher I, Mockly JF, Franco D, Fernandez H. [Peri-hepatitis abscess secondary to pelvic peritonitis]. J Gynecol Obstet Biol Reprod (Paris). 2002. 31:681–683.15. Marbet UA, Stalder GA, Vogtlin J, Loosli J, Frei A, Althaus B, et al. Diffuse peritonitis and chronic ascites due to infection with Chlamydia trachomatis in patients without liver disease: new presentation of the Fitz-Hugh-Curtis syndrome. Br Med J (Clin Res Ed). 1986. 293:5–6.16. Sam JW, Jacobs JE, Birnbaum BA. Spectrum of CT findings in acute pyogenic pelvic inflammatory disease. Radiographics. 2002. 22:1327–1334.17. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983. 148:839–843.18. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977. 33:159–174.19. Lopez-Zeno JA, Keith LG, Berger GS. The Fitz-Hugh-Curtis syndrome revisited. Changing perspectives after half a century. J Reprod Med. 1985. 30:567–582.20. Watanabe M, Tanaka S, Ono M, Hamamoto S, Niigaki M, Uchida Y, et al. Laparoscopic observations of hepatic capsular abnormalities: non-postoperative adhesions and hepatic capsular thickening. Gastrointest Endosc. 1999. 50:664–666.21. Romero-Gomez M, Corpas R, Sanchez-Munoz D, Grande L, Caballero V. Familial Mediterranean fever mimicking Fitz-Hugh-Curtis syndrome. Am J Gastroenterol. 2003. 98:701.22. Takeuchi H, Kitade M, Sakurai A, Kikuchi I, Kumakiri J, Kinoshita K. Fitz-Hugh and Curtis syndrome-like diaphragmatic endometriosis. Fertil Steril. 2005. 83:1039–1040.23. Romo LV, Clarke PD. Fitz-Hugh-Curtis Syndrome: pelvic inflammatory disease with an unusual CT presentation. J Comput Assist Tomogr. 1992. 16:832–833.24. Haight JB, Ockner SA. Chlamydia trachomatis perihepatitis with ascites. Am J Gastroenterol. 1988. 83:323–325.25. Fung GL, Silpa M. Fitz-Hugh and Curtis syndrome in a man. JAMA. 1981. 245:128.26. Kimball MW, Knee S. Gonococcal perihepatitis in a male. The Fitz-Hugh-Curtis syndrome. N Engl J Med. 1970. 282:1082–1084.27. Schoenwaelder M, Stuckey SL. Perihepatitis associated with systemic lupus erythematosus: computed tomography findings. Australas Radiol. 2005. 49:179–181.28. Chen WP, Chen JH, Hwang JI, Tsai JW, Chen JS, Hung SW, et al. Spectrum of transient hepatic attenuation differences in biphasic helical CT. AJR Am J Roentgenol. 1999. 172:419–424.29. Yamashita K, Jin MJ, Hirose Y, Morikawa M, Sumioka H, Itoh K, et al. CT finding of transient focal increased attenuation of the liver adjacent to the gallbladder in acute cholecystitis. AJR Am J Roentgenol. 1995. 164:343–346.30. Itai Y, Murata S, Kurosaki Y. Straight border sign of the liver: spectrum of CT appearances and causes. Radiographics. 1995. 15:1089–1102.31. Rodriguez E, Pombo F. Peritoneal tuberculosis versus peritoneal carcinomatosis: distinction based on CT findings. J Comput Assist Tomogr. 1996. 20:269–272.32. Quiroga S, Sebastia C, Pallisa E, Castella E, Perez-Lafuente M, Alvarez-Castells A. Improved diagnosis of hepatic perfusion disorders: value of hepatic arterial phase imaging during helical CT. Radiographics. 2001. 21:65–81. questionnaire 288-294.33. Yilmaz T, Sever A, Gur S, Killi RM, Elmas N. CT findings of abdominal tuberculosis in 12 patients. Comput Med Imaging Graph. 2002. 26:321–325.34. Zeitoun D, Brancatelli G, Colombat M, Federle MP, Valla D, Wu T, et al. Congenital hepatic fibrosis: CT findings in 18 adults. Radiology. 2004. 231:109–116.35. Muller-Schoop JW, Wang SP, Munzinger J, Schlapfer HU, Knoblauch M, Tammann RW. Chlamydia trachomatis as possible cause of peritonitis and perihepatitis in young women. Br Med J. 1978. 1:1022–1024.36. Katzman DK, Friedman IM, McDonald CA, Litt IF. Chlamydia trachomatis Fitz-Hugh-Curtis syndrome without salpingitis in female adolescents. Am J Dis Child. 1988. 142:996–998.37. Sharma JB, Malhotra M, Arora R. Fitz-Hugh-Curtis syndrome as a result of genital tuberculosis: a report of three cases. Acta Obstet Gynecol Scand. 2003. 82:295–297.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of Fitz-Hugh-Curtis syndrome diagnosed by pelvic CT imaging prior to diagnostic laparoscopy
- A Case of Fitz-Hugh-Curtis syndrome in a male patient
- Two Cases of Fitz-Hugh-Curtis Syndrome in Acute Phase
- Inflammatory Pseudotumor in the Liver and Right Omentum Caused by Pelvic Inflammatory Disease: A Case Report
- A Case of Fitz-Hugh-Curtis Syndrome which have Direct Adhesion between the Liver Capsule and Adjacent Anterior abdominal Wall