Yonsei Med J.
2007 Jun;48(3):449-456. 10.3349/ymj.2007.48.3.449.
Squamous Metaplasia and BCL-6 in Pediatric Adenoid Accompanied by Otitis Media with Effusion
- Affiliations
-
- 1Department of Otolaryngology, College of Medicine, Kyung Hee University, Seoul, Korea. yeo2park@yahoo.co.kr
- 2Department of Obstetrics and Gynecology, College of Medicine, The Catholic University, Suwon, Korea.
- KMID: 724100
- DOI: http://doi.org/10.3349/ymj.2007.48.3.449
Abstract
- PURPOSE
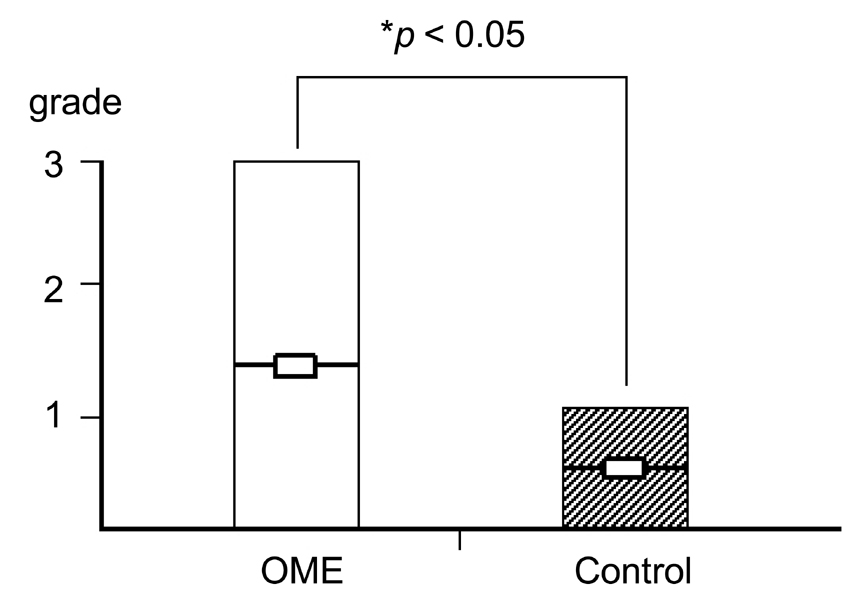
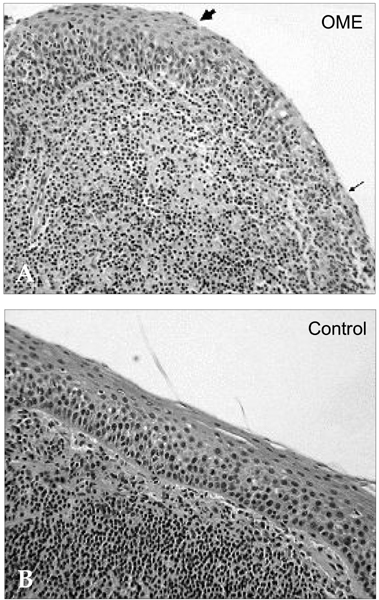
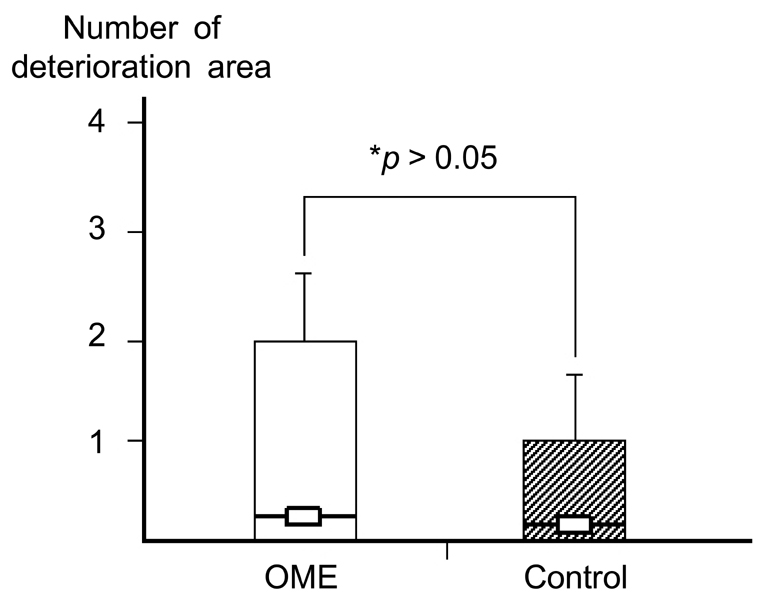
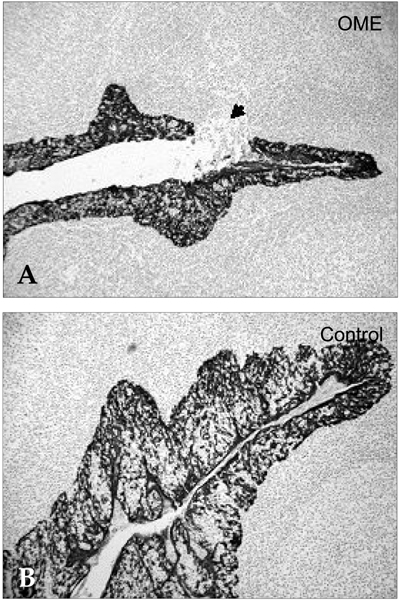
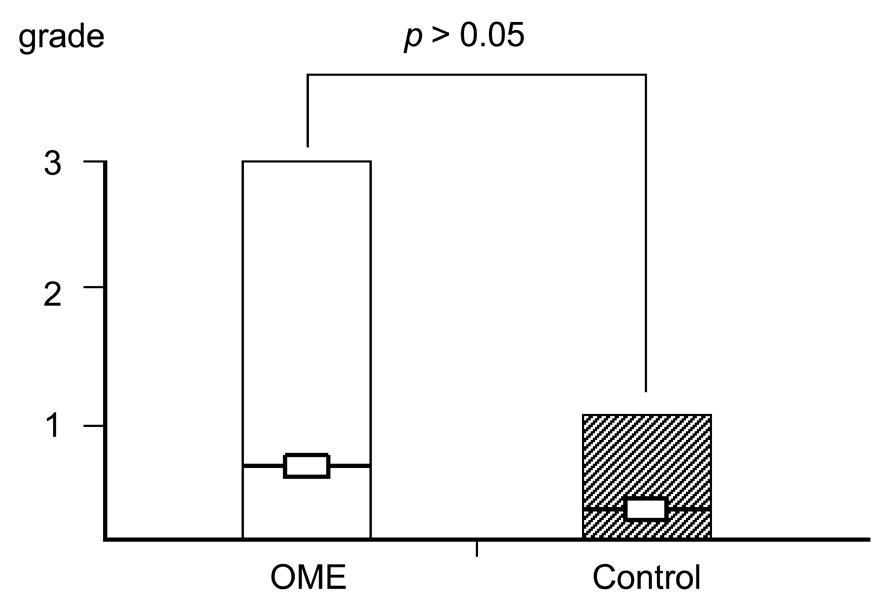
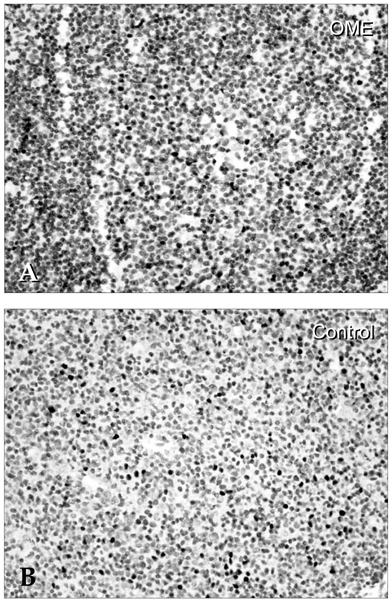
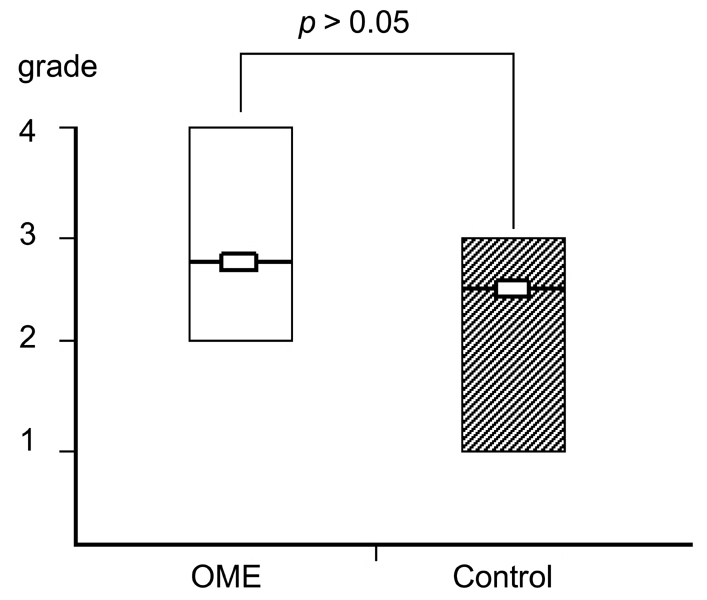
Deterioration of local immunity in the adenoids may make them vulnerable to infection by microorganisms, resulting in otitis media with effusion. To determine the factors associated with this condition, we evaluated adenoid size, mucosal barrier, squamous changes of ciliated epithelium, IgA secretion, and BCL-6 expression in adenoids. MATERIALS AND METHODS: Seventeen children diagnosed with otitis media with effusion (OME group) and 20 children without any history of OME (control group) were enrolled. Their adenoids were sized by lateral view X-ray and stained with hematoxylin and eosin to detect squamous metaplasia. The adenoids were also stained with cytokeratin to evaluate mucosal barriers, and with anti- IgA antibody and anti- BCL-6 antibody to determine expression of IgA and BCL-6. RESULTS: The OME group showed greater incidence of squamous metaplasia, fewer ciliated cells, and lower expression of BCL-6 (p < 0.05 each). Deterioration of the mucosal barrier was detected in the OME group (p > 0.05). IgA secretion and adenoid size were the same for the OME and the control groups. CONCLUSION: These results suggest that increased squamous metaplasia and lower BCL-6 expression in adenoids may be associated with increased susceptibility to OME.
Keyword
MeSH Terms
Figure
Reference
-
1. Clarke RW, De S. Otitis media in children. Hosp Med. 2005. 66:268–272.
Article2. Tomonaga K, Kurono Y, Chaen T, Mogi G. Adenoids and otitis media with effusion: nasopharyngeal flora. Am J Otolaryngol. 1989. 10:204–207.
Article3. Gates GA, Avery CA, Prihoda TJ. Effect of adenoidectomy upon children with chronic otitis media with effusion. Laryngoscope. 1988. 98:58–63.
Article4. Chu KC, Chang BC. Diagnosis and treatment of pediatric adenotonsillar disease. J Clin Otolaryngol. 1999. 10:134–146.
Article5. Perry M, Whyte A. Immunology of the tonsils. Immunol Today. 1998. 19:414–421.
Article6. Brodsky L, Koch RJ. Anatomic correlates of normal and diseased adenoids in children. Laryngoscope. 1992. 102:1268–1274.
Article7. Ophir D, Gilboa S, Halperin D, Marshak G. Obstructing adenoids in adolescents: changing trends? J Otolaryngol. 1993. 22:91–93.8. Fujioka M, Young LW, Girdany BR. Radiographic evaluation of adenoidal size in children: Adenoidal-nasopharyngeal ratio. AJR Am J Roentgenol. 1979. 133:401–404.
Article9. Paparella MM, Jung TT, Goycoolea MV. Paparella MM, Shumrick DA, Gluckman JL, Meyerhoff WL, editors. Secretory otitis media. Otolaryngology. 1991. 3rd ed. Philadelphia: Saunders;1317–1342.10. Post JC, Prestone RA, Aul JJ, Larkins-Pettigrew M, Rydquist-White J, Anderson KW, et al. Molecular analysis of bacterial pathogens in otitis media with effusion. JAMA. 1995. 273:1598–1604.
Article11. da Costa JL, Navarro A, Branco Neves J, Martin M. Otitis medias with effusion: association with the Eustachian tube dysfunction and adenoiditis. The case of the Central Hospital of Maputo. Acta Otorrinolaringol Esp. 2005. 56:290–294.
Article12. Maw AR. Chronic otitis media with effusion (glue ear) and adenotonsillectomy: prospective randomized controlled study. Br Med J (Clin Res Ed). 1983. 287:1586–1158.13. Gates GA, Avery CA, Prihoda TJ, Cooper JC Jr. Effectiveness of adenoidectomy and tympanostomy tubes in the treatment of chronic otitis media with effusion. N Engl J Med. 1987. 317:1444–1451.
Article14. Coyte PC, Croxford R, McIsaac W, Feldman W, Friedberg J. The role of adjuvant adenoidectomy and tonsillectomy in the outcome of the insertion of tympanostomy tubes. N Engl J Med. 2001. 344:1188–1195.
Article15. Maw AR, Bawden R. Factors affecting resolution of otitis media with effusion in children. Clin Otolaryngol Allied Sci. 1994. 19:125–130.
Article16. Roydhouse N. Adenoidectomy for otitis media with mucoid effusion. Ann Otol Rhinol Laryngol Suppl. 1980. 89:312–315.
Article17. Gates GA, Avery CA, Prihoda TJ. Effect of adenoidectomy upon children with chronic otitis media with effusion. Laryngoscope. 1988. 98:58–63.
Article18. Phillips DE, Maw AR, Harvey K. The nasopharynx and adenoid in children with glue ear compared with normal controls. Clin Otolaryngol Allied Sci. 1987. 12:255–260.
Article19. Maw AR, Parker A. Surgery of the tonsils and adenoids in relation to secretory otitis media in children. Acta Otolaryngol Suppl. 1988. 454:202–207.
Article20. Kamel RH, Ishak EA. Enlarged adenoid and adenoidectomy in adults: Endoscopic approach and histopathological study. J Laryngol Otol. 1990. 104:965–967.
Article21. Fujihara K, Fujihara T, Yamanaka N. Secretory IgA and squamous epithelization in adenoids of children with otitis media with effusion. Acta Otolaryngol Suppl. 1996. 523:155–157.22. Forsgren J, Rynnel-Dagoo B, Christensson B. In situ analysis of the immune microenvironment of the adenoid in children with and without secretory otitis media. Ann Otol Rhinol Laryngol. 1995. 104:189–196.
Article23. Brandtzaeg P. Immune functions and immunopathology of palatine and nasopharyngeal tonsils. Immunology of the Ear. 1992. New York: Raven;63–66.24. Arita M, Kodama S, Suzuki M, Mogi G. Single cell analysis of adenoid CD5+B cells and their protective contributions to nasopharyngeal immunity. Laryngoscope. 2003. 113:484–491.
Article25. Deweindt C, Albagli O, Bernardin F, Dhordain P, Quief S, Lantoine D, et al. The LAZ3/BCL-6 oncogene encodes a sequence-specific transcriptional inhibitor: a novel function for the BTB/ POZ domain as an autonomous repressing domain. Cell Growth Differ. 1995. 6:1495–1503.26. Chang CC, Ye BH, Chaganti RS, Dalla-Favera R. BCL-6, a POZ/zinc-finger protein, is a sequence-specific transcriptional repressor. Proc Natl Acad Sci U S A. 1996. 93:6947–6952.
Article27. Seyfert VL, Allman D, He Y, Staudt LM. Transcriptional repression by the proto-oncogene BCL-6. Oncogene. 1996. 12:2331–2342.28. Ye BH, Lista F, Lo Coco F, Knowles DM, Offit K, Chaganti RS, Dalla-Fevera R. Alterations of a zinc finger-encoding gene, BCL-6, in diffuse large-cell lymphoma. Science. 1993. 262:747–750.
Article29. Tumang JR, Frances R, Yeo SG, Rothstein TL. Spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J Immunol. 2005. 174:3173–3177.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Middle ear histopathology in children with otitis media with effusion
- Experimental otitis media with effusion induced by lipopolysaccharides from E. coli: the effects of endotoxin to the chronically of OME
- Detection of Bacteria in the Middle Ear Effusion and Adenoid Tissue of Chronic Otitis Media Patient Using PCR Method
- Prognostic significance of mastoid pneumatization in childhood otitis media with effusion: temporal bone CT evaluation
- An analysis of immunoglobulins and the role of allergy in otitis media with effusion