J Korean Med Sci.
2024 Nov;39(43):e320. 10.3346/jkms.2024.39.e320.
FGFR Inhibitors in Urothelial Cancer: From Scientific Rationale to Clinical Development
- Affiliations
-
- 1Department of Urology, Myongji Hospital, Hanyang University College of Medicine, Goyang, Korea
- KMID: 2561431
- DOI: http://doi.org/10.3346/jkms.2024.39.e320
Abstract
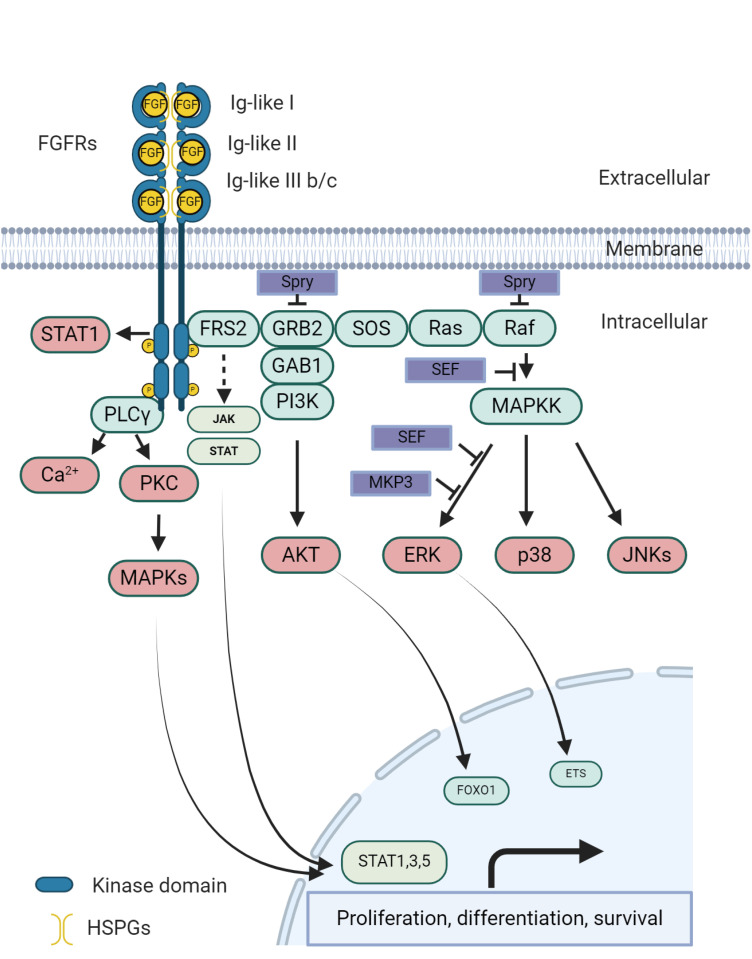
- In the past decade, the treatment of metastatic urothelial cancer (mUC), including bladder cancer (BC), has transformed significantly with the introduction of diverse therapies, such as immune checkpoint inhibitors, targeted therapies, and antibody-drug conjugates. This change is partly due to advancements in genomic understanding, particularly nextgeneration sequencing, which has identified numerous mutations in UC. Among these therapies, erdafitinib, a pan-fibroblast growth factor receptor (FGFR) inhibitor for specific FGFR2 and FGFR3 alterations, is the only targeted therapy approved till now. In 2019, erdafitinib became pivotal for the treatment of mUC, particularly in patients with specific FGFR alterations. Recent studies have highlighted the benefits of combining erdafitinib with immunotherapy, thereby broadening the treatment options. Ongoing investigations exist on its use in non-muscle-invasive BC and in combination with drugs such as enfortumab vedotin in mUC. Other FGFR-targeted agents are under development; however, overcoming FGFR resistance and ensuring the safety of combination therapies remain major hurdles. FGFR3 mutations are particularly prevalent in BC, a heterogeneous form of UC, and account for a considerable proportion of new cancer diagnoses annually. Approximately half of these cancers have FGFR3 mutations, with gene rearrangements being a common feature. These FGFR3 genomic alterations often occur independently of mutations in other BC oncogenes, such as TP53 and RB1. This review emphasizes the importance of FGFR inhibition in UC and the optimization of its use in clinical practice. Moreover, it underscores the ongoing efforts to evaluate combination strategies and early treatment testing to enhance the effectiveness of targeted therapies for UC.
Keyword
Figure
Reference
-
1. Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder cancer: a review. JAMA. 2020; 324(19):1980–1991. PMID: 33201207.2. Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. Epidemiology of bladder cancer. Med Sci (Basel). 2020; 8(1):15. PMID: 32183076.3. Halaseh SA, Halaseh S, Alali Y, Ashour ME, Alharayzah MJ. A review of the etiology and epidemiology of bladder cancer: all you need to know. Cureus. 2022; 14(7):e27330. PMID: 36042998.4. Kang MJ, Jung KW, Bang SH, Choi SH, Park EH, Yun EH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2020. Cancer Res Treat. 2023; 55(2):385–399. PMID: 36915245.5. International Agency for Research on Cancer. Global cancer observatory. Cancer today. Updated 2024. Accessed August 21, 2024. https://gco.iarc.fr/en .6. Han SH, Yuk HD. Epidemiology of urologic cancer in Korea: nationwide trends in the last 2 decades. Korean J Urol Oncol. 2023; 21(1):32–44.7. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: a global overview and recent trends. Eur Urol. 2017; 71(1):96–108. PMID: 27370177.8. Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin. 2020; 70(5):404–423. PMID: 32767764.9. Marei HE, Hasan A, Pozzoli G, Cenciarelli C. Cancer immunotherapy with immune checkpoint inhibitors (ICIs): potential, mechanisms of resistance, and strategies for reinvigorating T cell responsiveness when resistance is acquired. Cancer Cell Int. 2023; 23(1):64. PMID: 37038154.10. Kwon WA, Lee SY, Jeong TY, Kim HH, Lee MK. Antibody-drug conjugates in urothelial cancer: from scientific rationale to clinical development. Cancers (Basel). 2024; 16(13):2420. PMID: 39001482.11. Xu T, Xu W, Zheng Y, Li X, Cai H, Xu Z, et al. Comprehensive FGFR3 alteration-related transcriptomic characterization is involved in immune infiltration and correlated with prognosis and immunotherapy response of bladder cancer. Front Immunol. 2022; 13:931906. PMID: 35958598.12. Chioni AM, Grose RP. Biological significance and targeting of the FGFR axis in cancer. Cancers (Basel). 2021; 13(22):5681. PMID: 34830836.13. National Comprehensive Cancer Network (NCCN). NCCN gudelines for bladder cancer. Updated 2024. Accessed August 21, 2024. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1417 .14. Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med. 2024; 390(10):875–888. PMID: 38446675.15. Yun YR, Won JE, Jeon E, Lee S, Kang W, Jo H, et al. Fibroblast growth factors: biology, function, and application for tissue regeneration. J Tissue Eng. 2010; 2010(1):218142. PMID: 21350642.16. Xie Y, Su N, Yang J, Tan Q, Huang S, Jin M, et al. FGF/FGFR signaling in health and disease. Signal Transduct Target Ther. 2020; 5(1):181. PMID: 32879300.17. Itoh N. The Fgf families in humans, mice, and zebrafish: their evolutional processes and roles in development, metabolism, and disease. Biol Pharm Bull. 2007; 30(10):1819–1825. PMID: 17917244.18. Porębska N, Latko M, Kucińska M, Zakrzewska M, Otlewski J, Opaliński Ł. Targeting cellular trafficking of fibroblast growth factor receptors as a strategy for selective cancer treatment. J Clin Med. 2018; 8(1):7. PMID: 30577533.19. Krook MA, Reeser JW, Ernst G, Barker H, Wilberding M, Li G, et al. Fibroblast growth factor receptors in cancer: genetic alterations, diagnostics, therapeutic targets and mechanisms of resistance. Br J Cancer. 2021; 124(5):880–892. PMID: 33268819.20. Ruan R, Li L, Li X, Huang C, Zhang Z, Zhong H, et al. Unleashing the potential of combining FGFR inhibitor and immune checkpoint blockade for FGF/FGFR signaling in tumor microenvironment. Mol Cancer. 2023; 22(1):60. PMID: 36966334.21. Helsten T, Elkin S, Arthur E, Tomson BN, Carter J, Kurzrock R. The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin Cancer Res. 2016; 22(1):259–267. PMID: 26373574.22. American Association for Cancer Research (AACR). AACR GENIE 13.0-public data guide. Updated 2022. Accessed August 21, 2024. https://www.aacr.org/wp-content/uploads/2023/03/13.0_data_guide-1.pdf .23. Kamoun A, de Reyniès A, Allory Y, Sjödahl G, Robertson AG, Seiler R, et al. A consensus molecular classification of muscle-invasive bladder cancer. Eur Urol. 2020; 77(4):420–433. PMID: 31563503.24. Thomas J, Sonpavde G. Molecularly targeted therapy towards genetic alterations in advanced bladder cancer. Cancers (Basel). 2022; 14(7):1795. PMID: 35406567.25. Chang MM, Wu SZ, Yang SH, Wu CC, Wang CY, Huang BM. FGF9/FGFR1 promotes cell proliferation, epithelial-mesenchymal transition, M2 macrophage infiltration and liver metastasis of lung cancer. Transl Oncol. 2021; 14(11):101208. PMID: 34438248.26. Yue S, Li Y, Chen X, Wang J, Li M, Chen Y, et al. FGFR-TKI resistance in cancer: current status and perspectives. J Hematol Oncol. 2021; 14(1):23. PMID: 33568192.27. Wu Q, Ellis H, Siravegna G, Michel AG, Norden BL, Fece de la Cruz F, et al. Landscape of clinical resistance mechanisms to FGFR inhibitors in FGFR2-altered cholangiocarcinoma. Clin Cancer Res. 2024; 30(1):198–208. PMID: 37843855.28. Tan TZ, Rouanne M, Tan KT, Huang RY, Thiery JP. Molecular subtypes of urothelial bladder cancer: results from a meta-cohort analysis of 2411 tumors. Eur Urol. 2019; 75(3):423–432. PMID: 30213523.29. Zheng J, Zhang W, Li L, He Y, Wei Y, Dang Y, et al. Signaling pathway and small-molecule drug discovery of FGFR: a comprehensive review. Front Chem. 2022; 10:860985. PMID: 35494629.30. van Rhijn BW, Mertens LS, Mayr R, Bostrom PJ, Real FX, Zwarthoff EC, et al. FGFR3 mutation status and FGFR3 expression in a large bladder cancer cohort treated by radical cystectomy: implications for anti-FGFR3 treatment? Eur Urol. 2020; 78(5):682–687. PMID: 32682615.31. Xiao JF, Caliri AW, Duex JE, Theodorescu D. Targetable pathways in advanced bladder cancer: FGFR signaling. Cancers (Basel). 2021; 13(19):4891. PMID: 34638374.32. Zhang P, Yue L, Leng Q, Chang C, Gan C, Ye T, et al. Targeting FGFR for cancer therapy. J Hematol Oncol. 2024; 17(1):39. PMID: 38831455.33. Robertson AG, Kim J, Al-Ahmadie H, Bellmunt J, Guo G, Cherniack AD, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017; 171(3):540–556.e25. PMID: 28988769.34. Seiler R, Ashab HA, Erho N, van Rhijn BW, Winters B, Douglas J, et al. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur Urol. 2017; 72(4):544–554. PMID: 28390739.35. Teo MY, Mota JM, Whiting KA, Li HA, Funt SA, Lee CH, et al. Fibroblast growth factor receptor 3 alteration status is associated with differential sensitivity to platinum-based chemotherapy in locally advanced and metastatic urothelial carcinoma. Eur Urol. 2020; 78(6):907–915. PMID: 32753285.36. Ibrahim T, Gizzi M, Bahleda R, Loriot Y. Clinical development of FGFR3 inhibitors for the treatment of urothelial cancer. Bladder Cancer. 2019; 5(2):87–102.37. Necchi A, Lo Vullo S, Raggi D, Gloghini A, Giannatempo P, Colecchia M, et al. Prognostic effect of FGFR mutations or gene fusions in patients with metastatic urothelial carcinoma receiving first-line platinum-based chemotherapy: results from a large, single-institution cohort. Eur Urol Focus. 2019; 5(5):853–856. PMID: 29525380.38. Sethakorn N, O’Donnell PH. Spectrum of genomic alterations in FGFR3: current appraisal of the potential role of FGFR3 in advanced urothelial carcinoma. BJU Int. 2016; 118(5):681–691. PMID: 27271022.39. Szymczyk J, Sluzalska KD, Materla I, Opalinski L, Otlewski J, Zakrzewska M. FGF/FGFR-dependent molecular mechanisms underlying anti-cancer drug resistance. Cancers (Basel). 2021; 13(22):5796. PMID: 34830951.40. Lamont FR, Tomlinson DC, Cooper PA, Shnyder SD, Chester JD, Knowles MA. Small molecule FGF receptor inhibitors block FGFR-dependent urothelial carcinoma growth in vitro and in vivo. Br J Cancer. 2011; 104(1):75–82. PMID: 21119661.41. Rolfo C, Raez LE, Bronte G, Santos ES, Papadimitriou K, Buffoni L, et al. BIBF 1120/ nintedanib: a new triple angiokinase inhibitor-directed therapy in patients with non-small cell lung cancer. Expert Opin Investig Drugs. 2013; 22(8):1081–1088.42. Taylor MH, Lee CH, Makker V, Rasco D, Dutcus CE, Wu J, et al. Phase IB/II trial of lenvatinib plus pembrolizumab in patients with advanced renal cell carcinoma, endometrial cancer, and other selected advanced solid tumors. J Clin Oncol. 2020; 38(11):1154–1163. PMID: 31961766.43. Zheng X, Wang H, Deng J, Yao M, Zou X, Zhang F, et al. Safety and efficacy of the pan-FGFR inhibitor erdafitinib in advanced urothelial carcinoma and other solid tumors: a systematic review and meta-analysis. Front Oncol. 2023; 12:907377. PMID: 36776367.44. Subhan MA, Yalamarty SS, Filipczak N, Parveen F, Torchilin VP. Recent advances in tumor targeting via EPR effect for cancer treatment. J Pers Med. 2021; 11(6):571. PMID: 34207137.45. Li LY, Guo Y, Gonzalez M, Ouellet D. Effect of plasma protein binding on the pharmacokinetics of erdafitinib: results of an integrated cross-study analysis. J Clin Pharmacol. 2020; 60(3):391–399. PMID: 31602692.46. Bahleda R, Italiano A, Hierro C, Mita A, Cervantes A, Chan N, et al. Multicenter phase I study of erdafitinib (JNJ-42756493), oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced or refractory solid tumors. Clin Cancer Res. 2019; 25(16):4888–4897. PMID: 31088831.47. Peng J, Sridhar S, Siefker-Radtke AO, Selvarajah S, Jiang DM. Targeting the FGFR pathway in urothelial carcinoma: the future is now. Curr Treat Options Oncol. 2022; 23(9):1269–1287. PMID: 35962938.48. Janssen Inc. Product monograph including patient medication information. Updated 2021. Accessed August 21, 2024. https://pdf.hres.ca/dpd_pm/00063651.PDF .49. Loriot Y, Necchi A, Park SH, Garcia-Donas J, Huddart R, Burgess E, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019; 381(4):338–348. PMID: 31340094.50. U.S. Food and Drug Administration (FDA). FDA grants accelerated approval to erdafitinib for metastatic urothelial carcinoma. Updated 2019. Accessed August 21, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-erdafitinib-metastatic-urothelial-carcinoma .51. Loriot Y, Matsubara N, Park SH, Huddart RA, Burgess EF, Houede N, et al. Erdafitinib or chemotherapy in advanced or metastatic urothelial carcinoma. N Engl J Med. 2023; 389(21):1961–1971. PMID: 37870920.52. U.S. Food and Drug Administration (FDA). FDA approves erdafitinib for locally advanced or metastatic urothelial carcinoma. Updated 2019. Accessed August 21, 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-erdafitinib-locally-advanced-or-metastatic-urothelial-carcinoma .53. Qin Q, Patel V, Galsky MD. Urothelial carcinoma: the development of FGFR inhibitors in combination with immune checkpoint inhibitors. Expert Rev Anticancer Ther. 2020; 20(6):503–512. PMID: 32436413.54. Palakurthi S, Kuraguchi M, Zacharek SJ, Zudaire E, Huang W, Bonal DM, et al. The combined effect of FGFR inhibition and PD-1 blockade promotes tumor-intrinsic induction of antitumor immunity. Cancer Immunol Res. 2019; 7(9):1457–1471. PMID: 31331945.55. Benjamin DJ, Hsu R. Treatment approaches for FGFR-altered urothelial carcinoma: targeted therapies and immunotherapy. Front Immunol. 2023; 14:1258388. PMID: 37675102.56. Siefker-Radtke AO, Powles T, Moreno V, Kang TW, Cicin I, Girvin A, et al. Erdafitinib (ERDA) vs ERDA plus cetrelimab (ERDA+CET) for patients (pts) with metastatic urothelial carcinoma (mUC) and fibroblast growth factor receptor alterations (FGFRa): final results from the phase 2 Norse study. J Clin Oncol. 2023; 41(16):suppl. 4504.57. Rosenberg JE, Gajate P, Morales-Barrera R, Lee JL, Necchi A, Penel N, et al. Safety and efficacy of rogaratinib in combination with atezolizumab in cisplatin-ineligible patients (pts) with locally advanced or metastatic urothelial cancer (UC) and FGFR mRNA overexpression in the phase Ib/II FORT-2 study. J Clin Oncol. 2021; 39(15):suppl. 4521.58. Zengin ZB, Chehrazi-Raffle A, Salgia NJ, Muddasani R, Ali S, Meza L, et al. Targeted therapies: expanding the role of FGFR3 inhibition in urothelial carcinoma. Urol Oncol. 2022; 40(2):25–36. PMID: 34840077.59. Nadal R, Valderrama BP, Bellmunt J. Progress in systemic therapy for advanced-stage urothelial carcinoma. Nat Rev Clin Oncol. 2024; 21(1):8–27. PMID: 37945764.60. Shan KS, Dalal S, Thaw Dar NN, McLish O, Salzberg M, Pico BA. Molecular targeting of the fibroblast growth factor receptor pathway across various cancers. Int J Mol Sci. 2024; 25(2):849. PMID: 38255923.61. Wang H, Bo W, Feng X, Zhang J, Li G, Chen Y. Strategies and Recent Advances on Improving Efficient Antitumor of Lenvatinib Based on Nanoparticle Delivery System. Int J Nanomedicine. 2024; 19:5581–5603. PMID: 38882543.62. Casadei C, Dizman N, Schepisi G, Cursano MC, Basso U, Santini D, et al. Targeted therapies for advanced bladder cancer: new strategies with FGFR inhibitors. Ther Adv Med Oncol. 2019; 11:1758835919890285. PMID: 31803255.63. Siefker-Radtke AO, Currie G, Abella E, Vaena DA, Rezazadeh Kalebasty A, Curigliano G, et al. FIERCE-22: Clinical activity of vofatamab (V) a FGFR3 selective inhibitor in combination with pembrolizumab (P) in WT metastatic urothelial carcinoma, preliminary analysis. J Clin Oncol. 2019; 37(15):suppl. 4511.64. Kwon WA. PARP inhibitors in the treatment of prostate cancer: from scientific rationale to clinical development. World J Mens Health. 2024; 42(2):290–303. PMID: 37853532.65. Bourlon MT, Valdez P, Castro E. Development of PARP inhibitors in advanced prostate cancer. Ther Adv Med Oncol. 2024; 16:17588359231221337. PMID: 38205078.66. Yu SH, Kim SS, Kim S, Lee H, Kang TW. FGFR3 mutations in urothelial carcinoma: a single-center study using next-generation sequencing. J Clin Med. 2024; 13(5):1305. PMID: 38592174.67. Pietzak EJ, Bagrodia A, Cha EK, Drill EN, Iyer G, Isharwal S, et al. Next-generation sequencing of nonmuscle invasive bladder cancer reveals potential biomarkers and rational therapeutic targets. Eur Urol. 2017; 72(6):952–959. PMID: 28583311.68. Akanksha M, Sandhya S. Role of FGFR3 in urothelial carcinoma. Iran J Pathol. 2019; 14(2):148–155. PMID: 31528172.69. Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer. 2015; 15(1):25–41. PMID: 25533674.70. Nassar AH, Umeton R, Kim J, Lundgren K, Harshman L, Van Allen EM, et al. Mutational analysis of 472 urothelial carcinoma across grades and anatomic sites. Clin Cancer Res. 2019; 25(8):2458–2470. PMID: 30593515.71. Yuan X, Liu C, Wang K, Liu L, Liu T, Ge N, et al. The genetic difference between Western and Chinese urothelial cell carcinomas: infrequent FGFR3 mutation in Han Chinese patients. Oncotarget. 2016; 7(18):25826–25835. PMID: 27029078.72. Bou Zerdan M, Bratslavsky G, Jacob JM, Huang RS, Kravtsov O, Parimi V, et al. Landscape of fibroblast growth factor receptor (FGFR) genomic alterations (GA) in urothelial bladder cancer (UBC). J Clin Oncol. 2022; 40(16):suppl. 4568.73. Matsubara N, Osawa T, Abe T, Oya M, Nishimoto K, Iwahori T, et al. Real world experience of FGFR gene alterations and clinical outcomes in advanced/metastatic urothelial cancer in Japan: MONSTAR-SCREEN database study. J Clin Oncol. 2024; 42(4):suppl. 647.74. Matsubara N, Miura Y, Nishiyama H, Taoka R, Kojima T, Shimizu N, et al. Phase 3 THOR Japanese subgroup analysis: erdafitinib in advanced or metastatic urothelial cancer and fibroblast growth factor receptor alterations. Int J Clin Oncol. 2024; 29(10):1516–1527. PMID: 39017806.75. Katoh M, Loriot Y, Brandi G, Tavolari S, Wainberg ZA, Katoh M. FGFR-targeted therapeutics: clinical activity, mechanisms of resistance and new directions. Nat Rev Clin Oncol. 2024; 21(4):312–329. PMID: 38424198.76. Facchinetti F, Hollebecque A, Bahleda R, Loriot Y, Olaussen KA, Massard C, et al. Facts and new hopes on selective FGFR inhibitors in solid tumors. Clin Cancer Res. 2020; 26(4):764–774. PMID: 31585937.77. Vasseur D, Sassi H, Bayle A, Tagliamento M, Besse B, Marzac C, et al. Next-generation sequencing on circulating tumor DNA in advanced solid cancer: Swiss army knife for the molecular tumor board? A review of the literature focused on FDA approved test. Cells. 2022; 11(12):1901. PMID: 35741030.78. Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat Rev Cancer. 2017; 17(5):318–332. PMID: 28303906.79. Ellinghaus P, Neureiter D, Nogai H, Stintzing S, Ocker M. Patient selection approaches in FGFR inhibitor trials-many paths to the same end? Cells. 2022; 11(19):3180. PMID: 36231142.80. Ghosh S, Marrocco I, Yarden Y. Roles for receptor tyrosine kinases in tumor progression and implications for cancer treatment. Adv Cancer Res. 2020; 147:1–57. PMID: 32593398.81. Mahapatra S, Jonniya NA, Koirala S, Ursal KD, Kar P. The FGF/FGFR signalling mediated anti-cancer drug resistance and therapeutic intervention. J Biomol Struct Dyn. 2023; 41(22):13509–13533. PMID: 36995019.82. Maia MC, Salgia M, Pal SK. Harnessing cell-free DNA: plasma circulating tumour DNA for liquid biopsy in genitourinary cancers. Nat Rev Urol. 2020; 17(5):271–291. PMID: 32203306.83. Capuozzo M, Santorsola M, Bocchetti M, Perri F, Cascella M, Granata V, et al. p53: from fundamental biology to clinical applications in cancer. Biology (Basel). 2022; 11(9):1325. PMID: 36138802.84. Brandi G, Deiana C, Galvani L, Palloni A, Ricci AD, Rizzo A, et al. Are FGFR and IDH1-2 alterations a positive prognostic factor in intrahepatic cholangiocarcinoma? An unresolved issue. Front Oncol. 2023; 13:1137510. PMID: 37168376.85. Catto JW, Tran B, Rouprêt M, Gschwend JE, Loriot Y, Nishiyama H, et al. Erdafitinib in BCG-treated high-risk non-muscle-invasive bladder cancer. Ann Oncol. 2024; 35(1):98–106. PMID: 37871701.86. Vilaseca A, Guerrero F, Zainfeld D, Shore ND, Rodriguez Faba O, Meijer RP, et al. Safety and efficacy of the erdafitinib (erda) intravesical delivery system, TAR-210, in patients (pts) with non–muscle-invasive bladder cancer (NMIBC) or muscle-invasive bladder cancer (MIBC) harboring select FGFR mutations or fusions: phase 1 first-in-human study. J Clin Oncol. 2023; 41(6):suppl. TPS583.87. Zhou Y, Wu C, Lu G, Hu Z, Chen Q, Du X. FGF/FGFR signaling pathway involved resistance in various cancer types. J Cancer. 2020; 11(8):2000–2007. PMID: 32127928.88. Shi K, Wang G, Pei J, Zhang J, Wang J, Ouyang L, et al. Emerging strategies to overcome resistance to third-generation EGFR inhibitors. J Hematol Oncol. 2022; 15(1):94. PMID: 35840984.89. Sayegh N, Tripathi N, Agarwal N, Swami U. Clinical evidence and selecting patients for treatment with erdafitinib in advanced urothelial carcinoma. Onco Targets Ther. 2022; 15:1047–1055. PMID: 36186154.90. Siefker-Radtke AO, Matsubara N, Park SH, Huddart RA, Burgess EF, Özgüroğlu M, et al. Erdafitinib versus pembrolizumab in pretreated patients with advanced or metastatic urothelial cancer with select FGFR alterations: cohort 2 of the randomized phase III THOR trial. Ann Oncol. 2024; 35(1):107–117. PMID: 37871702.91. Catto JW, Tran B, Master VA, Roupret M, Pignot G, Tubaro A, et al. Phase 2 study of the efficacy and safety of erdafitinib in patients (pts) with bacillus Calmette-Guérin (BCG)-unresponsive, high-risk non–muscle-invasive bladder cancer (HR-NMIBC) with FGFR3/2 alterations (alt) in THOR-2: Cohort 2 interim analysis results. J Clin Oncol. 2023; 41(6):suppl. 503.92. Pal SK, Somford DM, Grivas P, Sridhar SS, Gupta S, Bellmunt J, et al. Targeting FGFR3 alterations with adjuvant infigratinib in invasive urothelial carcinoma: the phase III PROOF 302 trial. Future Oncol. 2022; 18(21):2599–2614. PMID: 35608106.93. Janssen Research & Development, LLC. A study of erdafitinib versus investigator choice of intravesical chemotherapy in participants who received Bacillus Calmette-Guérin (BCG) and recurred with high risk non-muscle-invasive bladder cancer (NMIBC). Updated 2024. Accessed August 21, 2024. https://clinicaltrials.gov/study/NCT04172675 .94. Janssen Research & Development, LLC. Study of erdafitinib intravesical delivery system for localized bladder cancer. Updated 2024. Accessed August 21, 2024. https://clinicaltrials.gov/study/NCT05316155 .95. National Cancer Institute (US). Testing combination erdafitinib and enfortumab vedotin in metastatic bladder cancer after treatment with chemotherapy and immunotherapy. Updated 2024. Accessed August 21, 2024. https://clinicaltrials.gov/study/NCT04963153. .96. Tyra Biosciences, Inc. Safety and preliminary anti-tumor activity of TYRA-300 in advanced urothelial carcinoma and other solid tumors with FGFR3 gene alterations (SURF301). Updated 2024. Accessed August 21, 2024. https://clinicaltrials.gov/study/NCT05544552. .97. Bayer. Phase 1b/2 study of rogaratinib (BAY1163877) in combination with atezolizumab in urothelial carcinoma (FORT-2). Updated 2024. Accessed August 21, 2024. https://clinicaltrials.gov/study/NCT03473756. .98. Abbisko Therapeutics Co, Ltd. A study to evaluate the safety and efficacy of AZD4547 combination with tislelizumab in patients with mUC. Updated 2023. Accessed August 21, 2024. https://clinicaltrials.gov/study/NCT05775874. .99. Eigl B. A study to compare the results of FGFR testing by either ctDNA blood testing or standard tumor tissue testing. Updated 2024. Accessed August 21, 2024. https://clinicaltrials.gov/study/NCT06129084 .100. Taiho Oncology, Inc. Futibatinib and pembrolizumab combination in the treatment of advanced or metastatic urothelial carcinoma. Updated 2024. Accessed August 21, 2024. https://clinicaltrials.gov/study/NCT04601857 .101. Kinnate Biopharma. A study to evaluate KIN-3248 in participants with advanced tumors harboring FGFR2 and//or FGFR3 gene alterations. Updated 2024. Accessed August 21, 2024. https://clinicaltrials.gov/study/NCT05242822 .102. Beijing InnoCare Pharma Tech Co., Ltd. A study of ICP-192 in patients with advanced solid tumors. Updated 2023. Accessed August 21, 2024. https://clinicaltrials.gov/study/NCT04565275 .103. Janssen Research & Development, LLC. A study of erdafitinib in participants with metastatic or locally advanced urothelial cancer. Updated 2024. Accessed August 21, 2024. https://clinicaltrials.gov/study/NCT03473743 .104. Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins. A study of pemigatinib in non-muscle invasive bladder cancer patients with recurrent low- or intermediate-risk tumors. Updated 2024. Accessed August 21, 2024. https://clinicaltrials.gov/study/NCT03914794 .105. Eli Lilly and Company. A study of LOXO-435 in participants with cancer with a change in a gene called FGFR3. Updated 2024. Accessed August 21, 2024. https://clinicaltrials.gov/study/NCT05614739 .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Fibroblast Growth Factor Receptors Inhibitors in Bladder Cancer
- The Development of Antibody-Drug Conjugates for Urothelial Carcinoma Treatment
- Perioperative immunotherapy in stage IB-III non-small cell lung cancer: a critical review of its rationale and considerations
- PARP Inhibitors in the Treatment of Prostate Cancer: From Scientific Rationale to Clinical Development
- Emerging agents for the treatment of metastatic urothelial cancer