Cancer Res Treat.
2024 Apr;56(2):624-633. 10.4143/crt.2023.1076.
Clinical Outcomes of Small Cell Carcinoma of the Genitourinary Tract and the Prognostic Significance of the Tumor Immune Microenvironment
- Affiliations
-
- 1Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Asan Institute of Life Sciences, Asan Medical Center, Seoul, Korea
- KMID: 2554351
- DOI: http://doi.org/10.4143/crt.2023.1076
Abstract
- Purpose
Small cell carcinoma of the genitourinary tract (GU SCC) is a rare disease with a poor prognosis. There are only limited treatment options due to insufficient understanding of the disease. In this study, we analyzed the clinical outcomes of patients with GU SCC and their association with the tumor immune phenotype.
Materials and Methods
Patients diagnosed with GU SCC were included. Survival outcomes according to the primary location (prostate and non-prostate) and stages (limited disease [LD] and extensive disease [ED]) were analyzed. We performed multiplex immunohistochemistry (IHC) in non-prostate SCC patients and analyzed the immune cell population.
Results
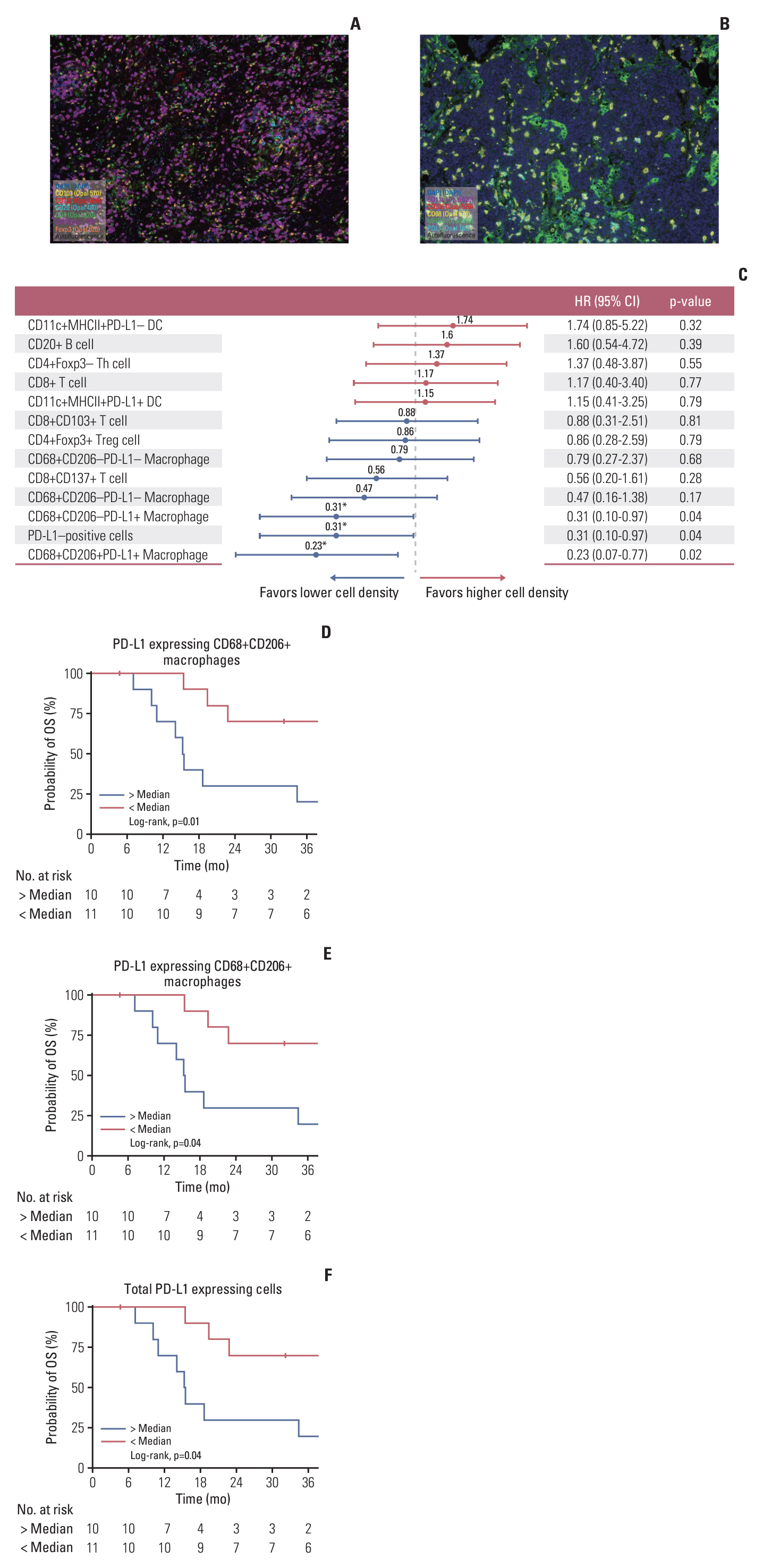
A total of 77 patients were included in this study. Their median age was 71 years, 67 patients (87.0%) were male, and 48 patients (62.3%) had non-prostate SCC. All patients with ED (n=31, 40.3%) received etoposide plus platinum (EP) as initial treatment and median overall survival (OS) was 9.7 months (95% confidence interval [CI], 7.1 to 18.6). Patients with LD (n=46, 59.7%) received EP followed by radiotherapy or surgery, and 24-months OS rate was 63.6% (95% CI, 49.9 to 81.0). The multiplex IHC analysis of 21 patients with non-prostate SCC showed that patients with a higher density of programmed death-ligand 1–expressing CD68+CD206+ M2-like macrophages had significantly worse OS outcomes with an adjusted hazards ratio of 4.17 (95% CI, 1.25 to 14.29; adjusted p=0.02).
Conclusion
Patients with GU SCC had a poor prognosis, even those with localized disease. The tumor immune phenotypes were significantly associated with survival. This finding provides new insights for treating GU SCC.
Keyword
Figure
Reference
-
References
1. Brennan SM, Gregory DL, Stillie A, Herschtal A, Mac Manus M, Ball DL. Should extrapulmonary small cell cancer be managed like small cell lung cancer? Cancer. 2010; 116:888–95.
Article2. Gupta K, Gupta S. Neuroendocrine differentiation in prostate cancer: key epigenetic players. Transl Cancer Res. 2017; 6:S104–8.
Article3. Thota S, Kistangari G, Daw H, Spiro T. A clinical review of small-cell carcinoma of the urinary bladder. Clin Genitourin Cancer. 2013; 11:73–7.
Article4. Lynch SP, Shen Y, Kamat A, Grossman HB, Shah JB, Millikan RE, et al. Neoadjuvant chemotherapy in small cell urothelial cancer improves pathologic downstaging and long-term outcomes: results from a retrospective study at the MD Anderson Cancer Center. Eur Urol. 2013; 64:307–13.
Article5. Ismaili N, Heudel PE, Elkarak F, Kaikani W, Bajard A, Ismaili M, et al. Outcome of recurrent and metastatic small cell carcinoma of the bladder. BMC Urol. 2009; 9:4.
Article6. Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018; 379:2220–9.
Article7. Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021; 22:51–65.8. Cicin I, Karagol H, Uzunoglu S, Uygun K, Usta U, Kocak Z, et al. Extrapulmonary small-cell carcinoma compared with small-cell lung carcinoma: a retrospective single-center study. Cancer. 2007; 110:1068–76.
Article9. Shen P, Jing Y, Zhang R, Cai MC, Ma P, Chen H, et al. Comprehensive genomic profiling of neuroendocrine bladder cancer pinpoints molecular origin and potential therapeutics. Oncogene. 2018; 37:3039–44.
Article10. Chang MT, Penson A, Desai NB, Socci ND, Shen R, Seshan VE, et al. Small-cell carcinomas of the bladder and lung are characterized by a convergent but distinct pathogenesis. Clin Cancer Res. 2018; 24:1965–73.
Article11. Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019; 51:202–6.
Article12. Koay EJ, Teh BS, Paulino AC, Butler EB. A Surveillance, Epidemiology, and End Results analysis of small cell carcinoma of the bladder: epidemiology, prognostic variables, and treatment trends. Cancer. 2011; 117:5325–33.
Article13. Siefker-Radtke AO, Dinney CP, Abrahams NA, Moran C, Shen Y, Pisters LL, et al. Evidence supporting preoperative chemotherapy for small cell carcinoma of the bladder: a retrospective review of the M. D. Anderson cancer experience. J Urol. 2004; 172:481–4.
Article14. Wang Y, Li Q, Wang J, Tong M, Xing H, Xue Y, et al. Small cell carcinoma of the bladder: the characteristics of molecular alterations, treatment, and follow-up. Med Oncol. 2019; 36:98.
Article15. Yamada Y, Beltran H. Clinical and biological features of neuroendocrine prostate cancer. Curr Oncol Rep. 2021; 23:15.
Article16. Papandreou CN, Daliani DD, Thall PF, Tu SM, Wang X, Reyes A, et al. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized smallcell carcinoma of the prostate. J Clin Oncol. 2002; 20:3072–80.
Article17. Husnain M, Park W, Ramos JC, Johnson TE, Chan J, Dasari A, et al. Complete response to ipilimumab and nivolumab therapy in a patient with extensive extrapulmonary highgrade small cell carcinoma of the pancreas and HIV infection. J Immunother Cancer. 2018; 6:66.
Article18. Ugwu JK, Nwanyanwu C, Shelke AR. Dramatic response of a metastatic primary small-cell carcinoma of the pancreas to a trial of immunotherapy with nivolumab: a case report. Case Rep Oncol. 2017; 10:720–5.
Article19. Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. 2021; 22:6995.
Article20. Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007; 26:373–400.
Article21. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012; 122:787–95.
Article22. Duan Z, Luo Y. Targeting macrophages in cancer immunotherapy. Signal Transduct Target Ther. 2021; 6:127.
Article23. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019; 19:133–50.
Article24. Lee JS, Ruppin E. Multiomics prediction of response rates to therapies to inhibit programmed cell death 1 and programmed cell death 1 ligand 1. JAMA Oncol. 2019; 5:1614–8.
Article25. Ganesan AP, Clarke J, Wood O, Garrido-Martin EM, Chee SJ, Mellows T, et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nat Immunol. 2017; 18:940–50.
Article26. Bianchini G, De Angelis C, Licata L, Gianni L. Treatment landscape of triple-negative breast cancer - expanded options, evolving needs. Nat Rev Clin Oncol. 2022; 19:91–113.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Crosstalk between tumor-associated macrophages and neighboring cells in hepatocellular carcinoma
- Immuno-oncology for B-cell lymphomas
- Epstein-Barr Virus-Associated Gastric Carcinoma and Specific Features of the Accompanying Immune Response
- The Tumor Immune Microenvironment in Cutaneous Squamous Cell Carcinoma Arising in Organ Transplant Recipients
- Clinical significance of measurements of serum CA19-9 in genitourinary cancer