Clin Endosc.
2024 Mar;57(2):164-174. 10.5946/ce.2023.074.
Role of contrast-enhanced harmonic endoscopic ultrasonography (EUS) and EUS elastography in pancreatic lesions
- Affiliations
-
- 1Second Department of Internal Medicine, Wakayama Medical University, Wakayama, Japan
- KMID: 2553751
- DOI: http://doi.org/10.5946/ce.2023.074
Abstract
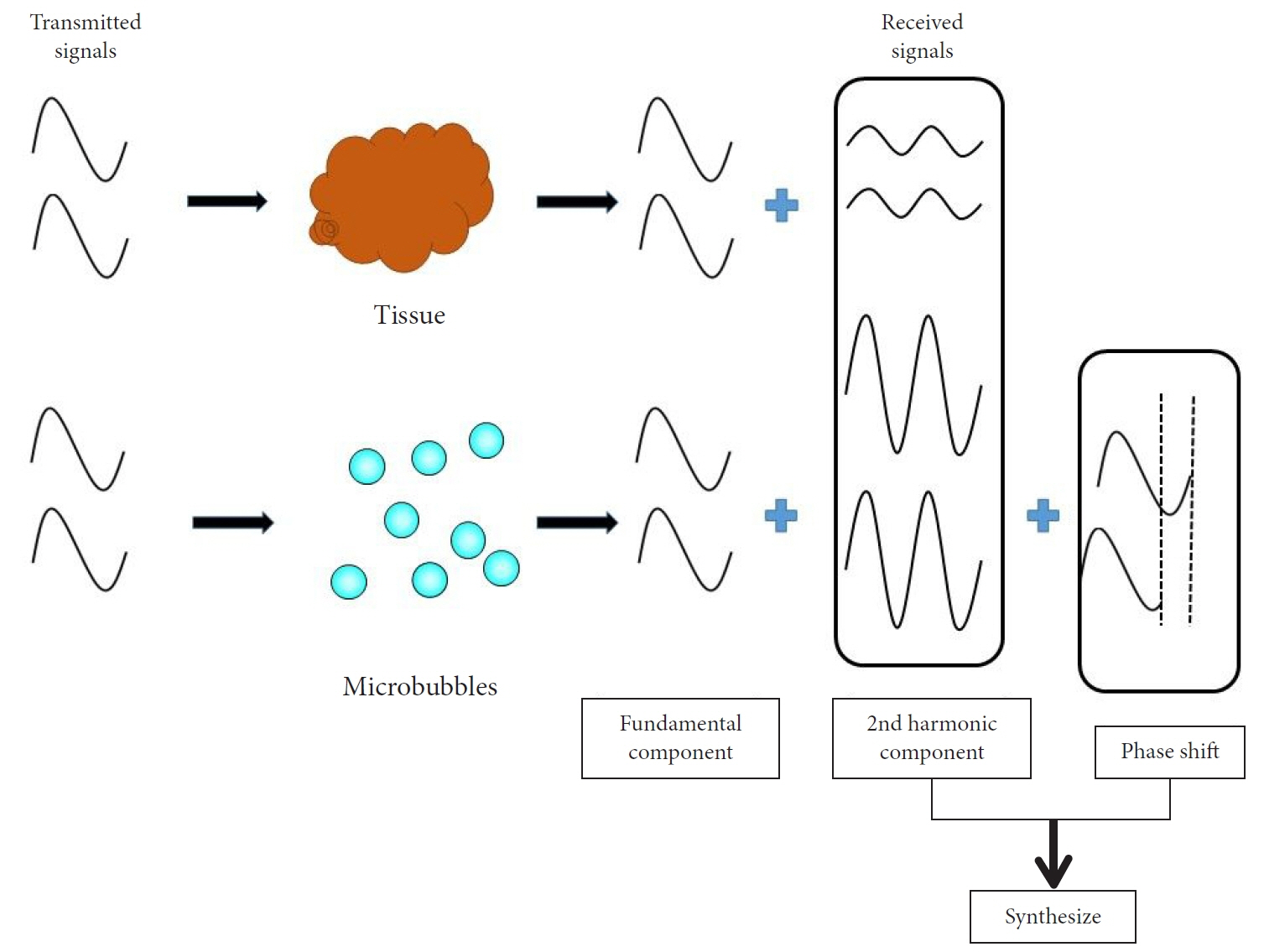
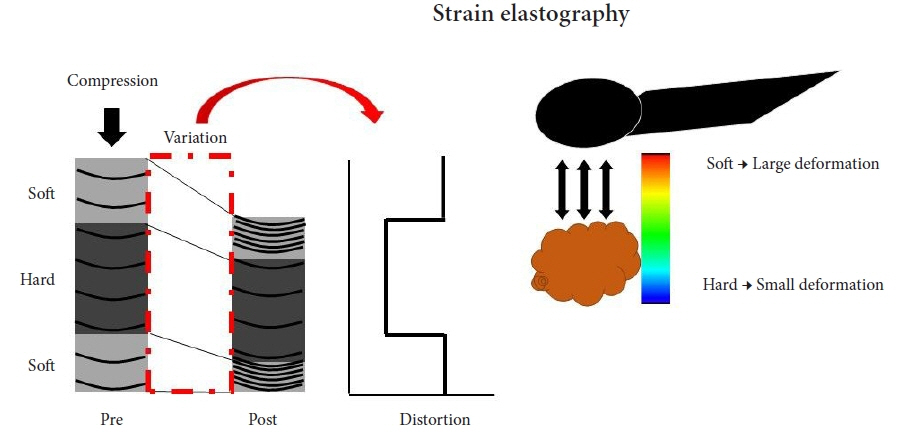
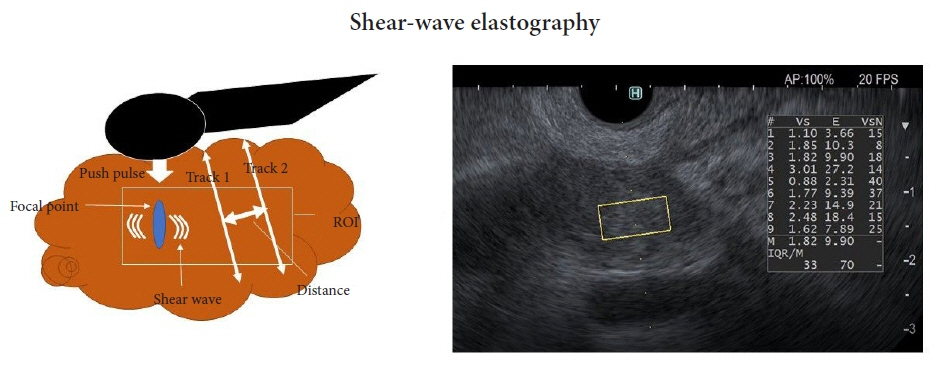
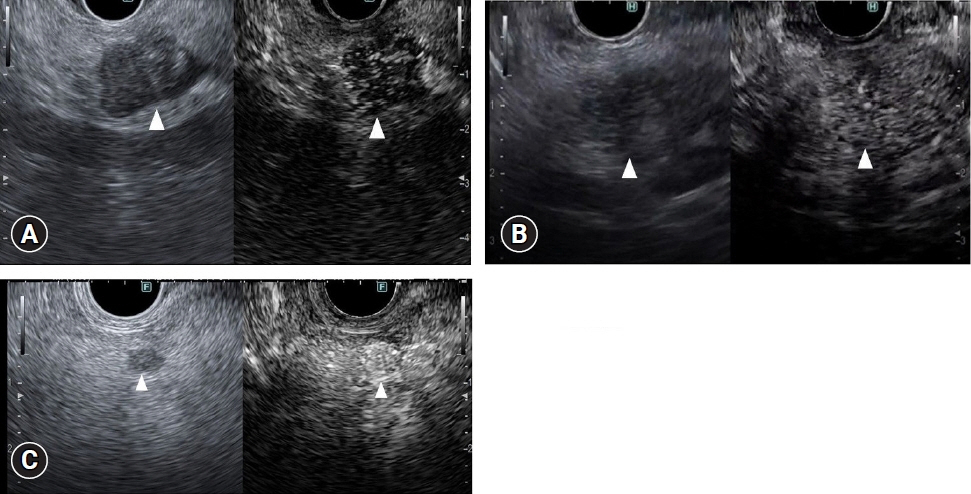
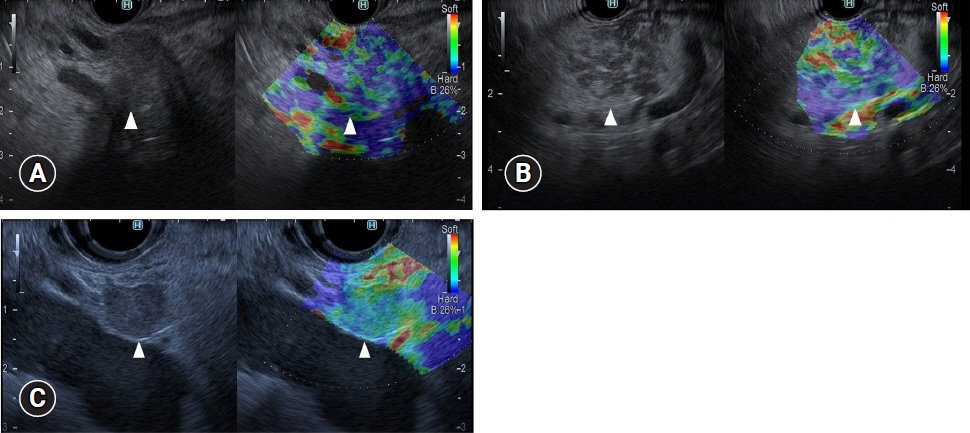
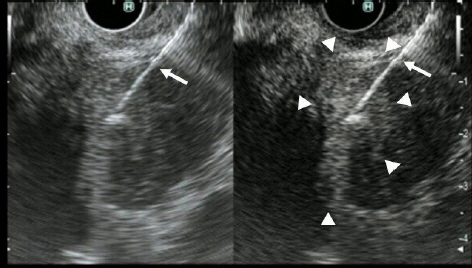
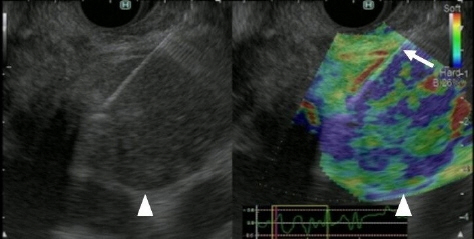
- Pancreatic cancers have a poor prognosis, and their incident rates have risen. Endoscopic ultrasonography (EUS) is an efficient and reliable diagnostic modality for pancreatic lesions, providing high spatial resolution. However, while EUS helps to detect minor pancreatic lesions, nearly all solid pancreatic lesions are hypoechoic, which creates difficulty in making differential diagnoses of pancreatic lesions. When diagnosing pancreatic lesions, the performance of image-enhanced EUS techniques is essential, such as EUS elastography or contrast-enhanced harmonic EUS (CH-EUS). CH-EUS diagnosis is based on assessing the vascularity of lesions, whereas tissue elasticity is measured via EUS elastography. Elastography is either strain or shear-wave, depending on the different mechanical properties being evaluated. The usefulness of enhanced EUS techniques is demonstrated in this review for the differential diagnosis of pancreatic lesions, including solid and cystic lesions, and pancreatic cancer staging.
Keyword
Figure
Reference
-
1. Sakamoto H, Kitano M, Suetomi Y, et al. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med Biol. 2008; 34:525–532.2. Kitano M, Kudo M, Maekawa K, et al. Dynamic imaging of pancreatic diseases by contrast enhanced coded phase inversion harmonic ultrasonography. Gut. 2004; 53:854–859.3. Yamashita Y, Tanioka K, Kawaji Y, et al. Utility of contrast-enhanced harmonic endoscopic ultrasonography for early diagnosis of small pancreatic cancer. Diagnostics (Basel). 2020; 10:23.4. Kamata K, Kitano M, Kudo M, et al. Value of EUS in early detection of pancreatic ductal adenocarcinomas in patients with intraductal papillary mucinous neoplasms. Endoscopy. 2014; 46:22–29.5. Kudo M. Contrast harmonic imaging in the diagnosis and treatment of hepatic tumors. Springer;2003. p. 22–30.6. Kitano M, Sakamoto H, Komaki T, et al. New techniques and future perspective of EUS for the differential diagnosis of pancreatic malignancies: contrast harmonic imaging. Dig Endosc. 2011; 23 Suppl 1:46–50.7. Kitano M, Sakamoto H, Matsui U, et al. A novel perfusion imaging technique of the pancreas: contrast-enhanced harmonic EUS (with video). Gastrointest Endosc. 2008; 67:141–150.8. Sakamoto H, Kitano M, Kamata K, et al. Diagnosis of pancreatic tumors by endoscopic ultrasonography. World J Radiol. 2010; 2:122–134.9. Hirooka Y, Itoh A, Kawashima H, et al. Contrast-enhanced endoscopic ultrasonography in digestive diseases. J Gastroenterol. 2012; 47:1063–1072.10. Reddy NK, Ioncică AM, Săftoiu A, et al. Contrast-enhanced endoscopic ultrasonography. World J Gastroenterol. 2011; 17:42–48.11. Kitano M, Kudo M, Yamao K, et al. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012; 107:303–310.12. Yamashita Y, Kato J, Ueda K, et al. Contrast-enhanced endoscopic ultrasonography for pancreatic tumors. Biomed Res Int. 2015; 2015:491782.13. Yamashita Y, Shimokawa T, Napoléon B, et al. Value of contrast-enhanced harmonic endoscopic ultrasonography with enhancement pattern for diagnosis of pancreatic cancer: a meta-analysis. Dig Endosc. 2019; 31:125–133.14. Chantarojanasiri T, Hirooka Y, Kawashima H, et al. Endoscopic ultrasound in diagnosis of solid pancreatic lesions: elastography or contrast-enhanced harmonic alone versus the combination. Endosc Int Open. 2017; 5:E1136–E1143.15. Iglesias-Garcia J, Larino-Noia J, Abdulkader I, et al. EUS elastography for the characterization of solid pancreatic masses. Gastrointest Endosc. 2009; 70:1101–1108.16. Zhang B, Zhu F, Li P, et al. Endoscopic ultrasound elastography in the diagnosis of pancreatic masses: a meta-analysis. Pancreatology. 2018; 18:833–840.17. Ohno E, Kawashima H, Ishikawa T, et al. Diagnostic performance of endoscopic ultrasonography-guided elastography for solid pancreatic lesions: shear-wave measurements versus strain elastography with histogram analysis. Dig Endosc. 2021; 33:629–638.18. Tanaka M, Fernández-Del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017; 17:738–753.19. Yamashita Y, Ueda K, Itonaga M, et al. Usefulness of contrast-enhanced endoscopic sonography for discriminating mural nodules from mucous clots in intraductal papillary mucinous neoplasms: a single-center prospective study. J Ultrasound Med. 2013; 32:61–68.20. Harima H, Kaino S, Shinoda S, et al. Differential diagnosis of benign and malignant branch duct intraductal papillary mucinous neoplasm using contrast-enhanced endoscopic ultrasonography. World J Gastroenterol. 2015; 21:6252–6260.21. Yamamoto N, Kato H, Tomoda T, et al. Contrast-enhanced harmonic endoscopic ultrasonography with time-intensity curve analysis for intraductal papillary mucinous neoplasms of the pancreas. Endoscopy. 2016; 48:26–34.22. Lisotti A, Napoleon B, Facciorusso A, et al. Contrast-enhanced EUS for the characterization of mural nodules within pancreatic cystic neoplasms: systematic review and meta-analysis. Gastrointest Endosc. 2021; 94:881–889.23. Kamata K, Takenaka M, Omoto S, et al. Impact of avascular areas, as measured by contrast-enhanced harmonic EUS, on the accuracy of FNA for pancreatic adenocarcinoma. Gastrointest Endosc. 2018; 87:158–163.24. Itonaga M, Kitano M, Kojima F, et al. The usefulness of EUS-FNA with contrast-enhanced harmonic imaging of solid pancreatic lesions: a prospective study. J Gastroenterol Hepatol. 2020; 35:2273–2280.25. Facciorusso A, Mohan BP, Crinò SF, et al. Contrast-enhanced harmonic endoscopic ultrasound-guided fine-needle aspiration versus standard fine-needle aspiration in pancreatic masses: a meta-analysis. Expert Rev Gastroenterol Hepatol. 2021; 15:821–828.26. Omoto S, Kitano M, Fukasawa M, et al. Tissue harmonic versus contrast-enhanced harmonic endoscopic ultrasonography for the diagnosis of pancreatic tumors: prospective multicenter study. Dig Endosc. 2022; 34:198–206.27. Kongkam P, Lakananurak N, Navicharern P, et al. Combination of EUS-FNA and elastography (strain ratio) to exclude malignant solid pancreatic lesions: a prospective single-blinded study. J Gastroenterol Hepatol. 2015; 30:1683–1689.28. Gheorghiu M, Sparchez Z, Rusu I, et al. Direct comparison of elastography endoscopic ultrasound fine-needle aspiration and B-mode endoscopic ultrasound fine-needle aspiration in diagnosing solid pancreatic lesions. Int J Environ Res Public Health. 2022; 19:1302.29. Imazu H, Uchiyama Y, Matsunaga K, et al. Contrast-enhanced harmonic EUS with novel ultrasonographic contrast (Sonazoid) in the preoperative T-staging for pancreaticobiliary malignancies. Scand J Gastroenterol. 2010; 45:732–738.30. Saffarian A, Eslami P, Dooghaie Moghadam A, et al. The potential of endoscopic ultrasound sonography (EUS)-elastography in determining the stage of pancreatic tumor. Gastroenterol Hepatol Bed Bench. 2021; 14:215–220.31. Miyata T, Kitano M, Omoto S, et al. Contrast-enhanced harmonic endoscopic ultrasonography for assessment of lymph node metastases in pancreatobiliary carcinoma. World J Gastroenterol. 2016; 22:3381–3391.32. Giovannini M, Thomas B, Erwan B, et al. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009; 15:1587–1593.33. Minaga K, Kitano M, Nakai A, et al. Improved detection of liver metastasis using Kupffer-phase imaging in contrast-enhanced harmonic EUS in patients with pancreatic cancer (with video). Gastrointest Endosc. 2021; 93:433–441.34. Emori T, Nuta J, Kawaji Y, et al. Value of contrast-enhanced harmonic endoscopic ultrasound for diagnosing hepatic metastases of pancreatic cancer: a prospective study. J Gastroenterol Hepatol. 2021; 36:3402–3409.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Status of Endoscopic Ultrasound Techniques for Pancreatic Neoplasms
- Contrast Harmonic Endoscopic Ultrasound in Pancreatic Diseases
- Clinical role of contrast-enhanced harmonic endoscopic ultrasound in differentiating pancreatic solid lesions
- New endoscopic ultrasonography techniques for pancreaticobiliary diseases
- EUS Elastography: Advances in Diagnostic EUS of the Pancreas