J Surg Ultrasound.
2023 Nov;10(2):32-41. 10.46268/jsu.2023.10.2.32.
Effects of Ultrasound Targeted Destruction of Echogenic Nanobubbles Containing Doxorubicin in Rat Liver
- Affiliations
-
- 1Department of Surgery, The Catholic University of Korea, Eunpyeong St. Mary’s Hospital, Seoul, Korea
- 2The Catholic University of Korea, College of Medicine, Seoul, Korea
- KMID: 2553148
- DOI: http://doi.org/10.46268/jsu.2023.10.2.32
Abstract
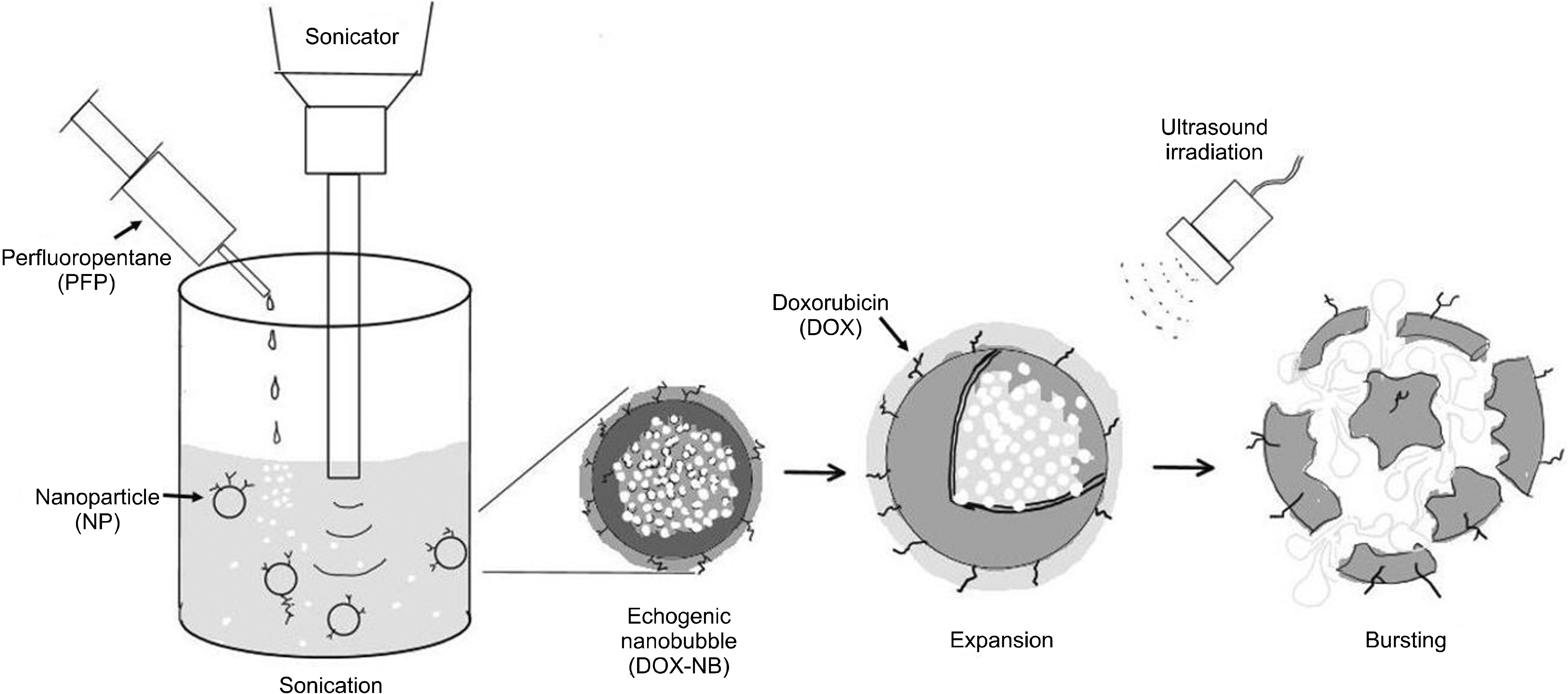
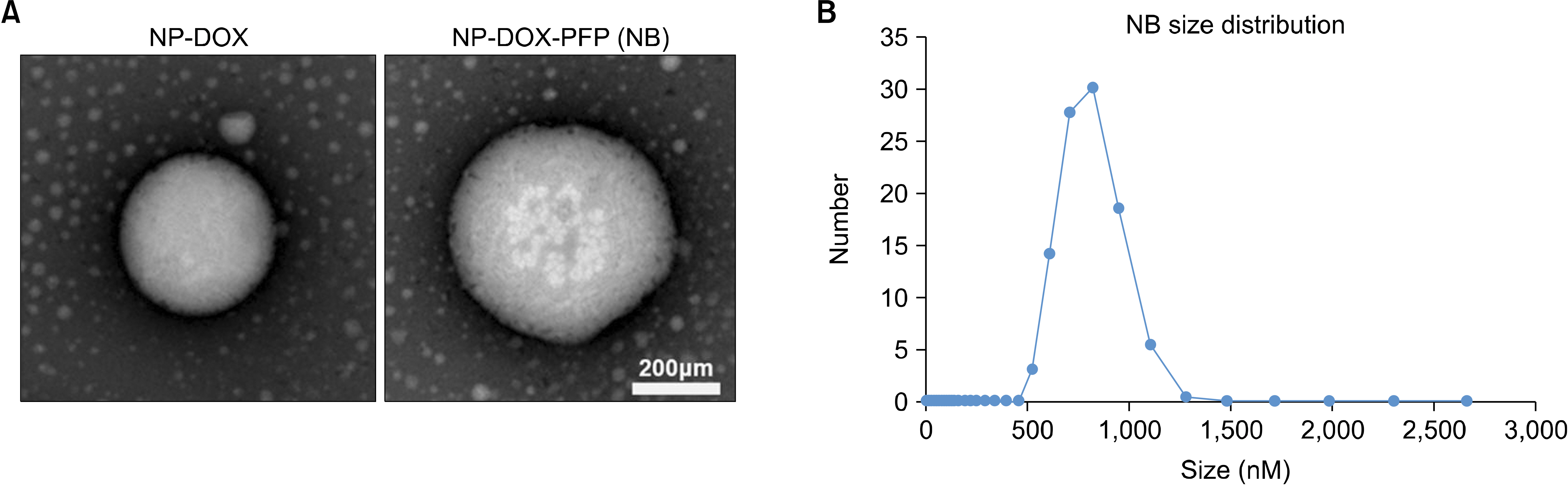
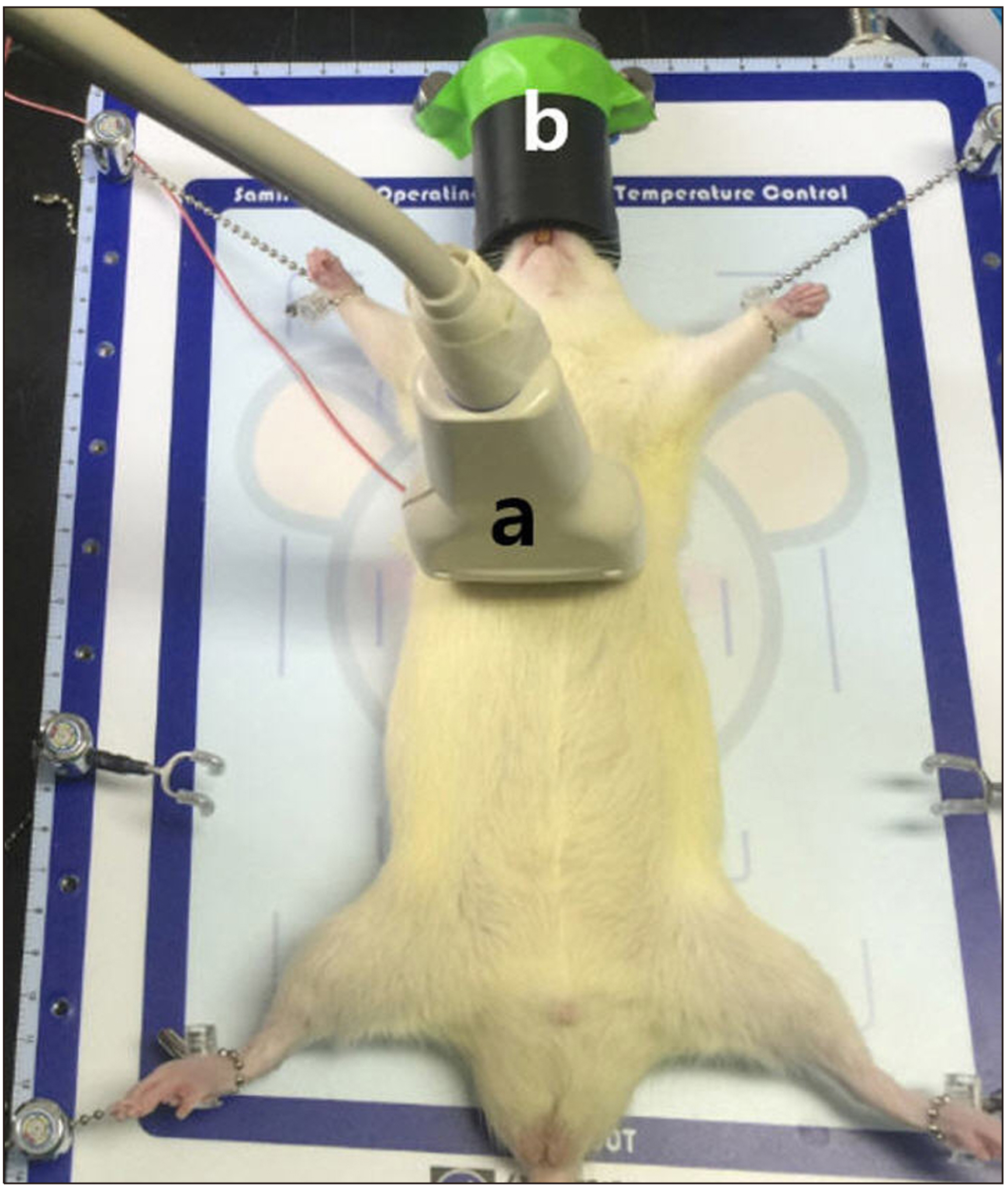
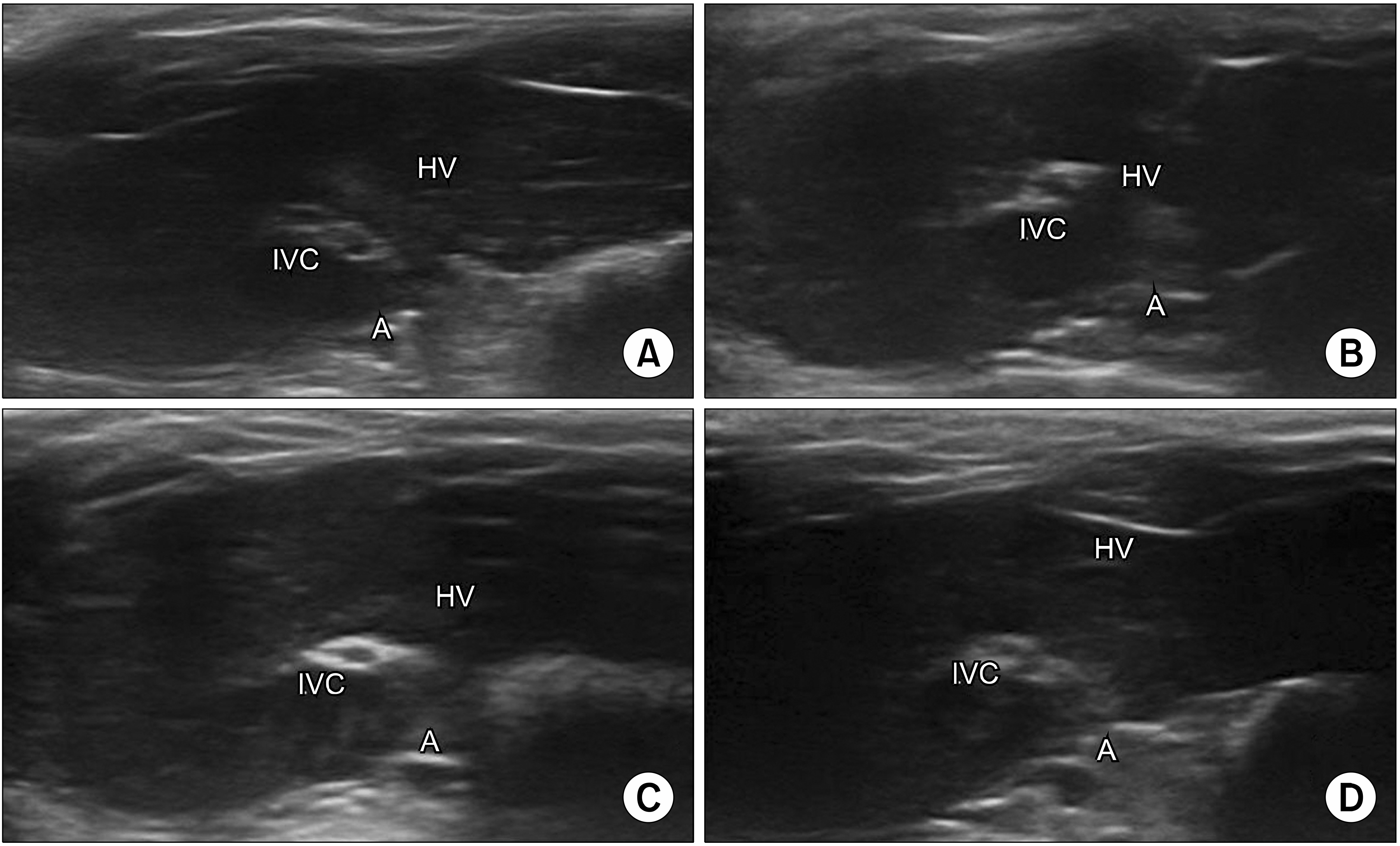
- Ultrasound is a widely used diagnostic medical imaging modality, and its therapeutic potential has recently been reported. This study examined whether ultrasound irradiation causes nanobubble destruction and identified the most effective frequency and duration of ultrasound for doxorubicin delivery in rat liver under ultrasound-targeted nanobubble destruction. Rats underwent an intravenous nanobubble injection via the caudal vein. An ultrasound probe was applied to the rat liver. The rats were divided into different groups based on the frequency. Fluorescence images were acquired using an IVIS® system. Laboratory tests were performed to determine the serum levels of AST (aspartate aminotransferase), ALT (alanine aminotransferase), total bilirubin, and creatinine. Under ultrasound irradiation, more nanobubbles were destroyed, and the doxorubicin uptake was increased in the rat liver (P < 0.05). The 5 MHz group showed an increased fluorescence efficiency in the liver (P < 0.05). Based on the duration of ultrasound irradiation, the 5, 10, and 13 MHz groups showed an increased fluorescence efficiency in the liver compared to the control group (P < 0.05). There was no significant difference between the 15- and 30-minute groups. The other organs showed no significant differences compared to the control group. The groups to which ultrasound was applied showed increased serum AST and ALT levels, but the effects did not last for 30 min. Ultrasound-targeted nanobubble destruction was effective for doxorubicin delivery in the rat liver. The most effective ultrasound frequency and irradiation duration were 5 MHz and 15 min, respectively.
Keyword
Figure
Reference
-
1. Juffermans LJ, Dijkmans PA, Musters RJ, van Wamel A, Bouakaz A, Ten Cate FJ, et al. 2004; Local drug and gene delivery through microbubbles and ultrasound: a safe and efficient alternative for viral vectors? Neth Heart J. 12:394–9.2. Xu J, Wu Y, Dong F. 2007; Clinical value of contrast-enhanced ultrasound in differentiating benign and malignant focal liver lesions. J Huazhong Univ Sci Technolog Med Sci. 27:703–5. DOI: 10.1007/s11596-007-0622-z. PMID: 18231748.
Article3. Yumita N, Nishigaki R, Umemura S. 2000; Sonodynamically induced antitumor effect of Photofrin II on colon 26 carcinoma. J Cancer Res Clin Oncol. 126:601–6. DOI: 10.1007/PL00008471. PMID: 11043398.
Article4. Liu P, Wang X, Zhou S, Hua X, Liu Z, Gao Y. 2011; Effects of a novel ultrasound contrast agent with long persistence on right ventricular pressure: comparison with SonoVue. Ultrasonics. 51:210–4. DOI: 10.1016/j.ultras.2010.07.008. PMID: 20825961.
Article5. Yang D, Tan KB, Gao YH, Liu H, Yang WX. 2012; Effects of diagnostic ultrasound-targeted microbubble destruction on permeability of normal liver in rats. Ultrasonics. 52:1065–71. DOI: 10.1016/j.ultras.2012.09.002. PMID: 23021237.
Article6. Wang G, Zhuo Z, Xia H, Zhang Y, He Y, Tan W, et al. 2013; Investigation into the impact of diagnostic ultrasound with microbubbles on the capillary permeability of rat hepatomas. Ultrasound Med Biol. 39:628–37. DOI: 10.1016/j.ultrasmedbio.2012.11.004. PMID: 23415284.
Article7. Chen ZY, Wang YX, Zhao YZ, Yang F, Liu JB, Lin Y, et al. 2014; Apoptosis induction by ultrasound and microbubble mediated drug delivery and gene therapy. Curr Mol Med. 14:723–36. DOI: 10.2174/1566524014666140804165245. PMID: 25088226.
Article8. Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. 2000; Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 65:271–84. DOI: 10.1016/S0168-3659(99)00248-5. PMID: 10699287.
Article9. Nguyen AT, Wrenn SP. 2014; Acoustically active liposome-nanobubble complexes for enhanced ultrasonic imaging and ultrasound-triggered drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 6:316–25. DOI: 10.1002/wnan.1255. PMID: 24459007.
Article10. Min HS, You DG, Son S, Jeon S, Park JH, Lee S, et al. 2015; Echogenic glycol chitosan nanoparticles for ultrasound-triggered cancer theranostics. Theranostics. 5:1402–18. DOI: 10.7150/thno.13099. PMID: 26681985. PMCID: PMC4672021.
Article11. El-Dakdouki MH, Xia J, Zhu DC, Kavunja H, Grieshaber J, O'Reilly S, et al. 2014; Assessing the in vivo efficacy of doxorubicin loaded hyaluronan nanoparticles. ACS Appl Mater Interfaces. 6:697–705. DOI: 10.1021/am404946v. PMID: 24308364. PMCID: PMC3912576.
Article12. Sastry CS, Lingeswara Rao JS. 1996; Determination of doxorubicin hydrochloride by visible spectrophotometry. Talanta. 43:1827–35. DOI: 10.1016/0039-9140(96)01932-7. PMID: 18966670.
Article13. Kremkau FW. 1979; Cancer therapy with ultrasound: a historical review. J Clin Ultrasound. 7:287–300. DOI: 10.1002/jcu.1870070410. PMID: 112118.
Article14. Chen H, Hwang JH. 2013; Ultrasound-targeted microbubble destruction for chemotherapeutic drug delivery to solid tumors. J Ther Ultrasound. 1:10. DOI: 10.1186/2050-5736-1-10. PMID: 25512858. PMCID: PMC4265893.
Article15. Lin CY, Huang YL, Li JR, Chang FH, Lin WL. 2010; Effects of focused ultrasound and microbubbles on the vascular permeability of nanoparticles delivered into mouse tumors. Ultrasound Med Biol. 36:1460–9. DOI: 10.1016/j.ultrasmedbio.2010.06.003. PMID: 20800173.
Article16. Mizushige K, Kondo I, Ohmori K, Hirao K, Matsuo H. 1999; Enhancement of ultrasound-accelerated thrombolysis by echo contrast agents: dependence on microbubble structure. Ultrasound Med Biol. 25:1431–7. DOI: 10.1016/S0301-5629(99)00095-2. PMID: 10626631.
Article17. Vedantham K, Swet JH, McKillop IH, El-Ghannam A. 2012; Evaluation of a bioresorbable drug delivery system for the treatment of hepatocellular carcinoma. J Biomed Mater Res A. 100:432–40. DOI: 10.1002/jbm.a.33228. PMID: 22105845.
Article18. Lencioni R. 2012; Chemoembolization for hepatocellular carci-noma. Semin Oncol. 39:503–9. DOI: 10.1053/j.seminoncol.2012.05.004. PMID: 22846867.
Article19. May BJ, Murthy R, Madoff DC. 2012; What's new in transarterial therapies for hepatocellular carcinoma? Gastrointest Cancer Res. 5(3 Suppl 1):S14–9.20. Cochran MC, Eisenbrey JR, Soulen MC, Schultz SM, Ouma RO, White SB, et al. 2011; Disposition of ultrasound sensitive polymeric drug carrier in a rat hepatocellular carcinoma model. Acad Radiol. 18:1341–8. DOI: 10.1016/j.acra.2011.06.013. PMID: 21971256. PMCID: PMC3188389.
Article21. Bekeredjian R, Kroll RD, Fein E, Tinkov S, Coester C, Winter G, et al. 2007; Ultrasound targeted microbubble destruction increases capillary permeability in hepatomas. Ultrasound Med Biol. 33:1592–8. DOI: 10.1016/j.ultrasmedbio.2007.05.003. PMID: 17618040.
Article22. Yin T, Wang P, Zheng R, Zheng B, Cheng D, Zhang X, et al. 2012; Nanobubbles for enhanced ultrasound imaging of tumors. Int J Nanomedicine. 7:895–904. DOI: 10.2147/IJN.S28830. PMID: 22393289. PMCID: PMC3289446.23. Kabalnov A, Klein D, Pelura T, Schutt E, Weers J. 1998; Dissolution of multicomponent microbubbles in the bloodstream: 1. Theory. Ultrasound Med Biol. 24:739–49. DOI: 10.1016/S0301-5629(98)00034-9. PMID: 9695277.
Article24. Klibanov AL. 2006; Microbubble contrast agents: targeted ultrasound imaging and ultrasound-assisted drug-delivery applications. Invest Radiol. 41:354–62. DOI: 10.1097/01.rli.0000199292.88189.0f. PMID: 16481920.25. Kim JH, Kim YS, Kim S, Park JH, Kim K, Choi K, et al. 2006; Hydrophobically modified glycol chitosan nanoparticles as carriers for paclitaxel. J Control Release. 111:228–34. DOI: 10.1016/j.jconrel.2005.12.013. PMID: 16458988.
Article26. Rahman A, Ganjei A, Neefe JR. 1986; Comparative immunotoxicity of free doxorubicin and doxorubicin encapsulated in cardiolipin liposomes. Cancer Chemother Pharmacol. 16:28–34. DOI: 10.1007/BF00255282. PMID: 3484382.
Article27. De Cock I, Zagato E, Braeckmans K, Luan Y, de Jong N, De Smedt SC, et al. 2015; Ultrasound and microbubble mediated drug delivery: acoustic pressure as determinant for uptake via mem-brane pores or endocytosis. J Control Release. 197:20–8. DOI: 10.1016/j.jconrel.2014.10.031. PMID: 25449801.
Article28. Li H, Wang J, Huang G, Wang P, Zheng R, Zhang C, et al. 2013; Multifunctionalized microbubbles for cancer diagnosis and therapy. Anticancer Agents Med Chem. 13:403–13. DOI: 10.2174/1871520611313030004. PMID: 23092268.
Article29. Tartis MS, McCallan J, Lum AF, LaBell R, Stieger SM, Matsunaga TO, et al. 2006; Therapeutic effects of paclitaxel-containing ultrasound contrast agents. Ultrasound Med Biol. 32:1771–80. DOI: 10.1016/j.ultrasmedbio.2006.03.017. PMID: 17112963.
Article30. Cai WB, Yang HL, Zhang J, Yin JK, Yang YL, Yuan LJ, et al. 2015; The optimized fabrication of nanobubbles as ultrasound contrast agents for tumor imaging. Sci Rep. 5:13725. DOI: 10.1038/srep13725. PMID: 26333917. PMCID: PMC4558543.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Ultrasound Targeted Destruction of Echogenic Nanobubbles Containing Doxorubicin in Rat Liver
- Echogenic Rim of Hepatic Hemangioma on Abdominal Ultrasound
- Echogenic liver abscess mimicking solid mass : sonographic characteristics
- Ultrasound manifestation of hepatocellular carcinoma
- Targeted Ultrasound Imaging of Apoptosis with Annexin A5 Microbubbles in Acute Doxorubicin-Induced Cardiotoxicity