Ann Pediatr Endocrinol Metab.
2024 Feb;29(1):3-11. 10.6065/apem.2346206.103.
An overview of growth hormone therapy in pediatric cases documented in the Kabi International Growth Study (Pfizer International Growth Database)
- Affiliations
-
- 1The Saban Research Institute, Children’s Hospital Los Angeles, Keck School of Medicine of University of Southern California, Los Angeles, CA, USA
- 2Department of Pediatric Endocrinology, University Children´s Hospital, Tübingen, Germany
- 3Pfizer Inc., New York, NY, USA
- 4New York University Grossman School of Medicine, New York, NY, USA
- KMID: 2553012
- DOI: http://doi.org/10.6065/apem.2346206.103
Abstract
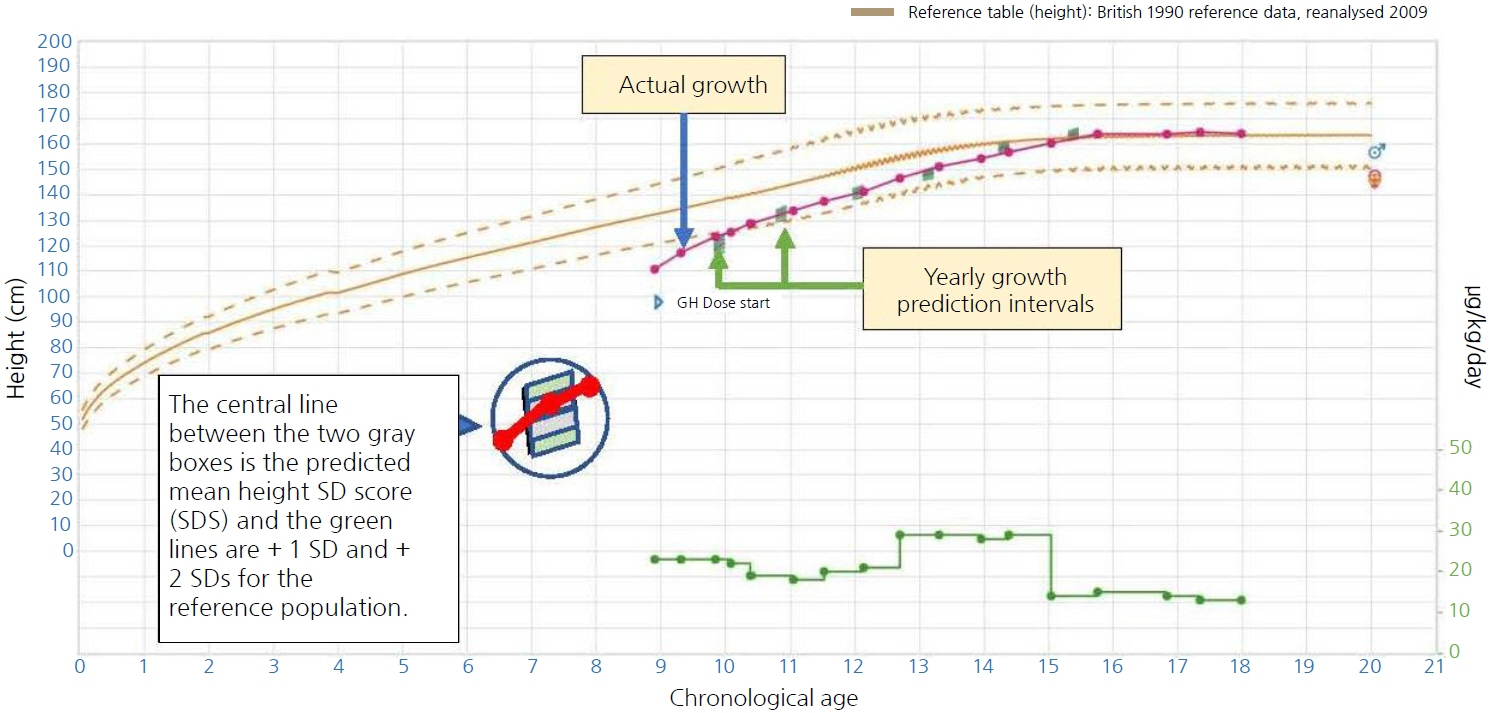
- The Kabi International Growth Study (KIGS) was first established in 1987 and is the largest pharmaco-epidemiological study of recombinant human growth hormone (rhGH). KIGS is aimed at evaluating long-term safety and treatment outcomes in pediatric subjects who received Genotropin rhGH therapy (Pfizer, New York, NY, USA) as prescribed by physicians in real-world clinical practice settings. KIGS data have been used to answer multiple research questions related to growth, growth prediction, and growth hormone treatment, leading to the publication of 129 peer-reviewed manuscripts and 24 biannual reports, outcomes from 10 expert meetings, and 3 books. The KIGS has shown that rhGH is safe and increases both the short-term height gain and adult height in patients with GH deficiency (GHD) and multiple other non-GHD conditions associated with short stature.
Keyword
Figure
Cited by 1 articles
-
Commentary on "Somatrogon in pediatric growth hormone deficiency: a comprehensive review of clinical trials and real-world considerations"
Yoo-Mi Kim
Ann Pediatr Endocrinol Metab. 2025;30(1):1-2. doi: 10.6065/apem.2524129edi01.
Reference
-
References
1. Ranke MB, Wit JM. Growth hormone - past, present and future. Nat Rev Endocrinol. 2018; 14:285–300.2. Parks JS. Gene sequence and production. of recombinant MetGH/hGH. Pediatr Endocrinol Rev. 2018; 16(Suppl 1):17–27.3. Long Island University, Clinical Drug Experience Knowledgebase: Kabi Pharmacia [Internet]. Brookville (NY): Long Island University; 2003 [2022 Sep 1]. Available from: https://www.cdek.liu.edu/org/1112/.4. Wallström A, Trulsson L. The Kabi International Growth Study: rationale, organization, and development. In: Ranke MB, Gunnarsson R, editors. Progress in growth hormone therapy – 5 years of KIGS. Mannheim (Germany): J&J Verlag, 1994;1-9.5. Wilton P. KIGS: structure and organization. Growth hormone therapy in pediatrics. 20 years of KIGS. In: Ranke MB, Price DA, Reiter EO, editors. Basel (Switzerland): Karger, 2007:1-5.6. Non-interventional study abstract for external disclosure. Protocol A6281306 (87-052). KIGS® (Pfizer International Growth Database). New York (NY): Pfizer; 2014.7. Ranke MB. The KIGS aetiology classification system. Progress in growth hormone therapy – 5 years of KIGS. In: Ranke MB, Gunnarsson R, editors. Mannheim (Germany): J&J Verlag, 1994:51-61.8. Wilton P. Adverse events during growth hormone treatment: 5 years' experience in the Kabi International Growth Study. In: Ranke MB, Gunnarsson R, editors. Progress in growth hormone therapy – 5 years of KIGS. Mannheim (Germany): J&J Verlag, 1994:291-307.9. Maghnie M, Ranke MB, Geffner ME, Vlachopapadopoulou E, Ibáñez L, Carlsson M, et al. Safety and efficacy of pediatric growth hormone therapy: results from the full KIGS cohort. J Clin Endocrinol Metab. 2022; 107:3287–301.10. Ranke MB. Who stops growth hormone in KIGS - and why? Growth hormone therapy in pediatrics. 20 Years of KIGS. In: Ranke MB, Price DA, Reiter EO, editors. Basel (Switzerland): Karger, 2007:183-8.11. Gutiérrez LP, Kołtowska-Häggström M, Jönsson PJ, Mattsson AF, Svensson D, Westberg B, et al. Registries as a tool in evidence-based medicine: example of KIMS (Pfizer International Metabolic Database). Pharmacoepidemiol Drug Saf. 2008; 17:90–102.12. Johannsson G, Touraine P, Feldt-Rasmussen U, Pico A, Vila G, Mattsson AF, et al. Long-term safety of growth hormone in adults with growth hormone deficiency: overview of 15 809 GH-treated patients. J Clin Endocrinol Metab. 2022; 107:1906–19.13. Ranke MB, Lindberg A. Predicting growth in response to growth hormone treatment. Growth Horm IGF Res. 2009; 19:1–11.14. Ranke MB, Lindberg A. Predicting growth in response to growth hormone treatment – the KIGS approach. Growth hormone therapy in pediatrics. 20 Years of KIGS. In: Ranke MB, Price DA, Reiter EO, editors. Basel (Switzerland): Karger, 2007:422-31.15. Ranke MB, Lindberg A. Observed and predicted total pubertal growth during treatment with growth hormone in adolescents with idiopathic growth hormone deficiency, Turner syndrome, short stature, born small for gestational age and idiopathic short stature: KIGS analysis and review. Horm Res Paediatr. 2011; 75:423–32.16. Loftus J, Lindberg A, Aydin F, Gomez R, Maghnie M, Rooman R, et al. Individualised growth response optimisation (iGRO) tool: an accessible and easy-to-use growth prediction system to enable treatment optimisation for children treated with growth hormone. J Pediatr Endocrinol Metab. 2017; 30:1019–26.17. Geffner M, Lundberg M, Koltowska-Häggström M, Abs R, Verhelst J, Erfurth EM, et al. Changes in height, weight, and body mass index in children with craniopharyngioma after three years of growth hormone therapy: analysis of KIGS (Pfizer International Growth Database). J Clin Endocrinol Metab. 2004; 89:5435–40.18. Verhelst J, Kendall-Taylor P, Erfurth EM, Price DA, Geffner M, Koltowska-Häggström M, et al. Baseline characteristics and response to 2 years of growth hormone (GH) replacement of hypopituitary patients with GH deficiency due to adult-onset craniopharyngioma in comparison with patients with nonfunctioning pituitary adenoma: data from KIMS (Pfizer International Metabolic Database). J Clin Endocrinol Metab. 2005; 90:4636–43.19. Yuen KC, Koltowska-Häggström M, Cook DM, Fox JL, Jönsson PJ, Geffner ME, et al. Clinical characteristics and effects of GH replacement therapy in adults with childhood-onset craniopharyngioma compared with those in adults with other causes of childhood-onset hypothalamic-pituitary dysfunction. Eur J Endocrinol. 2013; 169:511–9.20. Yuen KC, Kołtowska-Häggström M, Cook DM, Fox JL, Jönsson PJ, Geffner ME, et al. Primary treatment regimen and diabetes insipidus as predictors of health outcomes in adults with childhood-onset craniopharyngioma. J Clin Endocrinol Metab. 2014; 99:1227–35.21. Yuen KCJ, Mattsson AF, Burman P, Erfurth EM, Camacho-Hubner C, Fox JL, et al. Relative risks of contributing factors to morbidity and mortality in adults with craniopharyngioma on growth hormone replacement. J Clin Endocrinol Metab. 2018; 103:768–77.22. Darendeliler F. Growth hormone treatment in rare disorders: the KIGS experience. 20 Years of KIGS. In: Ranke MB, Price DA, Reiter EO, editors. Basel (Switzerland): Karger, 2007:213-39.23. Ranke MB, Gunnarsson R, editors. Progress in growth hormone therapy – 5 years of KIGS. Mannheim (Germany): J&J Verlag, 1994:1-313.24. Ranke MB, Wilton P, editors. Growth hormone therapy in KIGS – 10 years experience. Leipzig (Germany): Johann Ambrosius Barth Verlag, 1999:1-409.25. Ranke MB, Price DA, Reiter EO, editors. Growth hormone therapy in pediatrics – 20 years of KIGS. Basel (Switzerland): Karger, 2007;1-511.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of yeast-derived methionyl recombinant growth hormone on growth hormone deficient dwarf
- Clinical effects of yeast derived recombinant methionyl growth hormone in growth hormone deficiency
- Growth Hormone Therapy in Intrauterine Growth Retardation(IOGR)
- Clinical effects of recombinant Korean growth hormone (LBD-003)
- The mechanism of Arginine-stimulated growth hormone secretion