Anesth Pain Med.
2024 Jan;19(1):35-43. 10.17085/apm.23107.
Application of the Bair Hugger™ core body temperature at wrist region with upper body warming blanket: a prospective observational study
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Chung-Ang University, Chung-Ang University Hospital, Seoul, Korea
- KMID: 2552855
- DOI: http://doi.org/10.17085/apm.23107
Abstract
- Background
Body temperature monitoring is essential during the perioperative period. However, core body temperature measurement requires invasive device that may cause complications. This study aimed to evaluate the accuracy of non-invasive Bair Hugger™ core body temperature monitoring system (BHTMS) at the wrist compared with esophageal temperature under general anesthesia.
Methods
Twenty adult patients of the American Society of Anesthesiologists physical status I or II were enrolled. BHTMS sensor was applied at wrist region. After tracheal intubation, an esophageal probe was inserted. Bair Hugger™ upper body warming blankets were used. Esophageal temperature (Teso) and BHTMS at wrist (Twrist) were recorded every 10 min.
Results
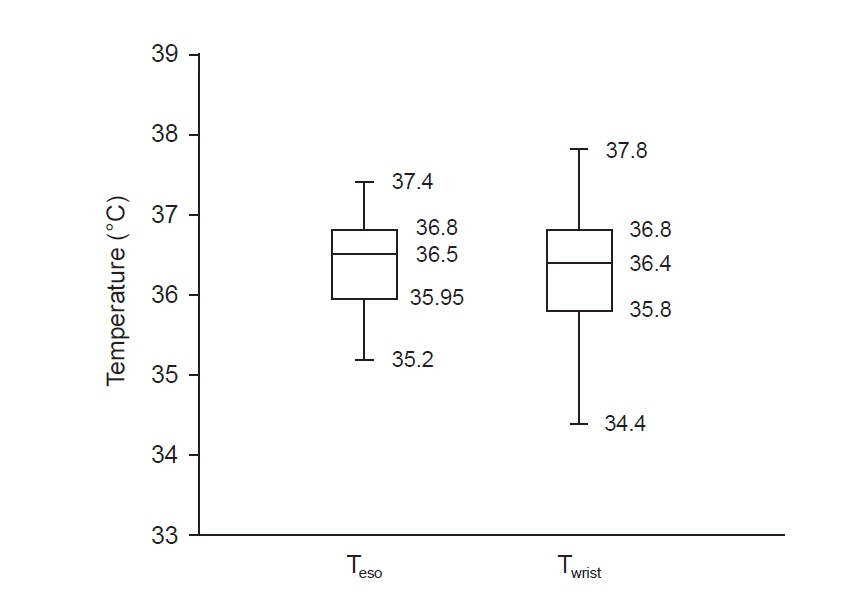
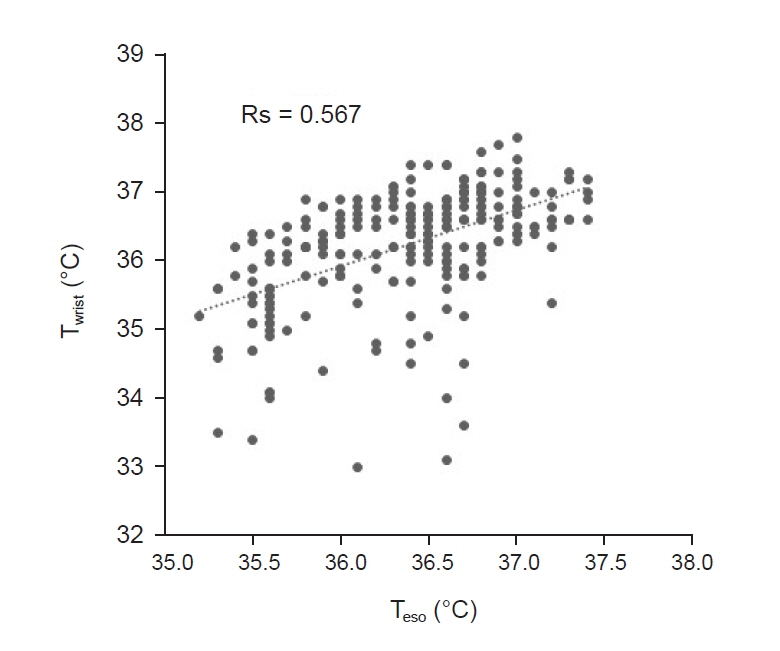
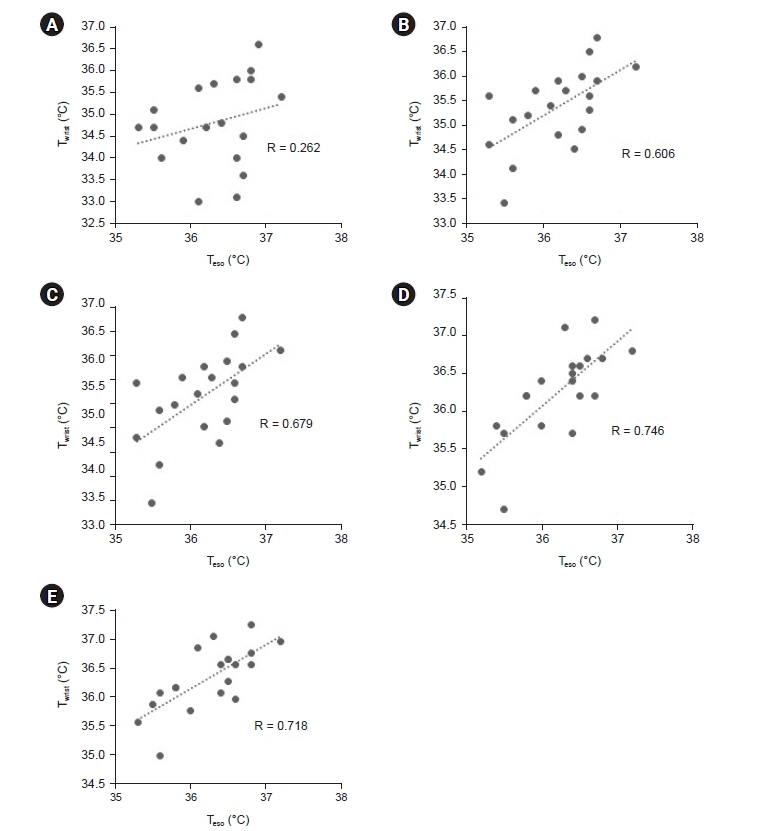
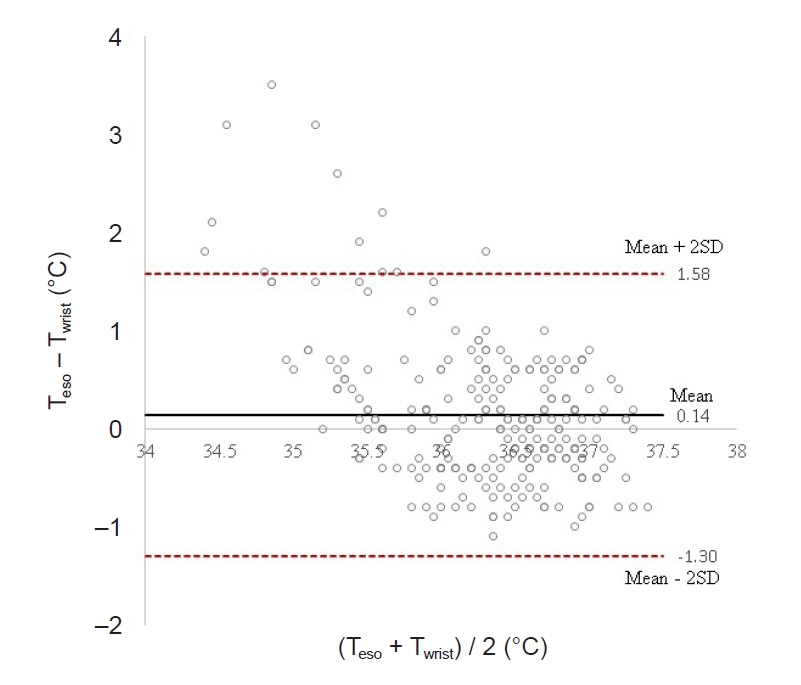
Total of 257 pairs of data sets were analyzed: Teso and Twrist had no statistically significant difference (P = 0.103). Median of Teso and Twrist were 36.5°C and 36.4°C. Bland-Altman analysis showed Teso – Twrist of 0.14°C ± 1.44. Subsequently, 99 pairs of 0–40 min data set were analyzed and showed significant difference at 0 and 10 min (P < 0.001) but no significant difference at 20, 30 and 40 min. Bland– Altman plot by times showed difference (Teso - Twrist) of 1.49°C ± 2.00, 0.82°C ± 1.30, 0.29°C ± 1.32, –0.03°C ± 0.84, and –0.12°C ± 0.82 at 0, 10, 20, 30 and 40 min respectively.
Conclusions
BHTMS at wrist area under the upper body warming blanket is a potential alternative other than esophageal temperature for monitoring body temperature after 30 min of anesthesia induction.
Keyword
Figure
Reference
-
1. Schmied H, Kurz A, Sessler DI, Kozek S, Reiter A. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet. 1996; 347:289–92.
Article2. Rohrer MJ, Natale AM. Effect of hypothermia on the coagulation cascade. Crit Care Med. 1992; 20:1402–5.
Article3. Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996; 334:1209–15.
Article4. Frank SM, Fleisher LA, Breslow MJ, Higgins MS, Olson KF, Kelly S, et al. Perioperative maintenance of normothermia reduces the incidence of morbid cardiac events. A randomized clinical trial. JAMA. 1997; 277:1127–34.
Article5. Hopkins PM. Malignant hyperthermia: advances in clinical management and diagnosis. Br J Anaesth. 2000; 85:118–28.
Article6. Rosenberg H, Davis M, James D, Pollock N, Stowell K. Malignant hyperthermia. Orphanet J Rare Dis. 2007; 2:21.
Article7. Severinghaus JW. Temperature gradients during hypothermia. Ann N Y Acad Sci. 1959; 80:515–21.
Article8. Hymczak H, Golab A, Mendrala K, Plicner D, Darocha T, Podsiadlo P, et al. Core temperature measurement-principles of correct measurement, problems, and complications. Int J Environ Res Public Health. 2021; 18:10606.
Article9. Fox RH, Solman AJ. A new technique for monitoring the deep body temperature in man from the intact skin surface. J Physiol. 1971; 212:8–10.10. Boisson M, Alaux A, Kerforne T, Mimoz O, Debaene B, Dahyot-Fizelier C, et al. Intra-operative cutaneous temperature monitoring with zero-heat-flux technique (3M SpotOn) in comparison with oesophageal and arterial temperature: a prospective observational study. Eur J Anaesthesiol. 2018; 35:825–30.11. Iden T, Horn EP, Bein B, Böhm R, Beese J, Höcker J. Intraoperative temperature monitoring with zero heat flux technology (3M SpotOn sensor) in comparison with sublingual and nasopharyngeal temperature: an observational study. Eur J Anaesthesiol. 2015; 32:387–91.12. Pesonen E, Silvasti-Lundell M, Niemi TT, Kivisaari R, Hernesniemi J, Mäkinen MT. The focus of temperature monitoring with zero-heat-flux technology (3M Bair-Hugger): a clinical study with patients undergoing craniotomy. J Clin Monit Comput. 2019; 33:917–23.
Article13. Eshraghi Y, Nasr V, Parra-Sanchez I, Van Duren A, Botham M, Santoscoy T, et al. An evaluation of a zero-heat-flux cutaneous thermometer in cardiac surgical patients. Anesth Analg. 2014; 119:543–9.
Article14. Tachibana S, Chida Y, Yamakage M. Using the Bair HuggerTM temperature monitoring system in neck and chest regions: a pilot study. JA Clin Rep. 2019; 5:32.15. Kang IG, Jeong WJ, Moon KM. The relationship between radial artery depth and wrist extension angle measured by ultrasonography. J Korean Soc Emerg Med. 2013; 24:279–83.16. Mekjavić IB, Rempel ME. Determination of esophageal probe insertion length based on standing and sitting height. J Appl Physiol (1985). 1990; 69:376–9.
Article17. Sessler DI. Perioperative heat balance. Anesthesiology. 2000; 92:578–96.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rewarming Intervention Program for Abdominal Surgery Patients
- The Effect of Pre and Intra-Operative Warming Therapy on Tympanic Temperature Changes during Perioperative Phase in Receiving Patients with Total Hip Replacement
- Comparison of Forced Air Warming and Radiant Heating on Body Temperature and Shivering of Post-operative Patients
- The Effects of Active Warming on Pain, Temperature, and Thermal Discomfort in Postoperative Patients after General Anesthesia for Abdominal Surgery
- The efficacy of warming blanket on reducing intraoperative hypothermia in patients undergoing transurethral resection of bladder tumor under general anesthesia