Lab Med Online.
2023 Oct;13(4):263-274. 10.47429/lmo.2023.13.4.263.
Technical Considerations and Miscellaneous Findings on Electrophoretograms of Serum Protein Capillary Electrophoresis
- Affiliations
-
- 1Department of Laboratory Medicine, University of Ulsan College of Medicine, Ulsan University Hospital, Ulsan, Korea
- KMID: 2552752
- DOI: http://doi.org/10.47429/lmo.2023.13.4.263
Abstract
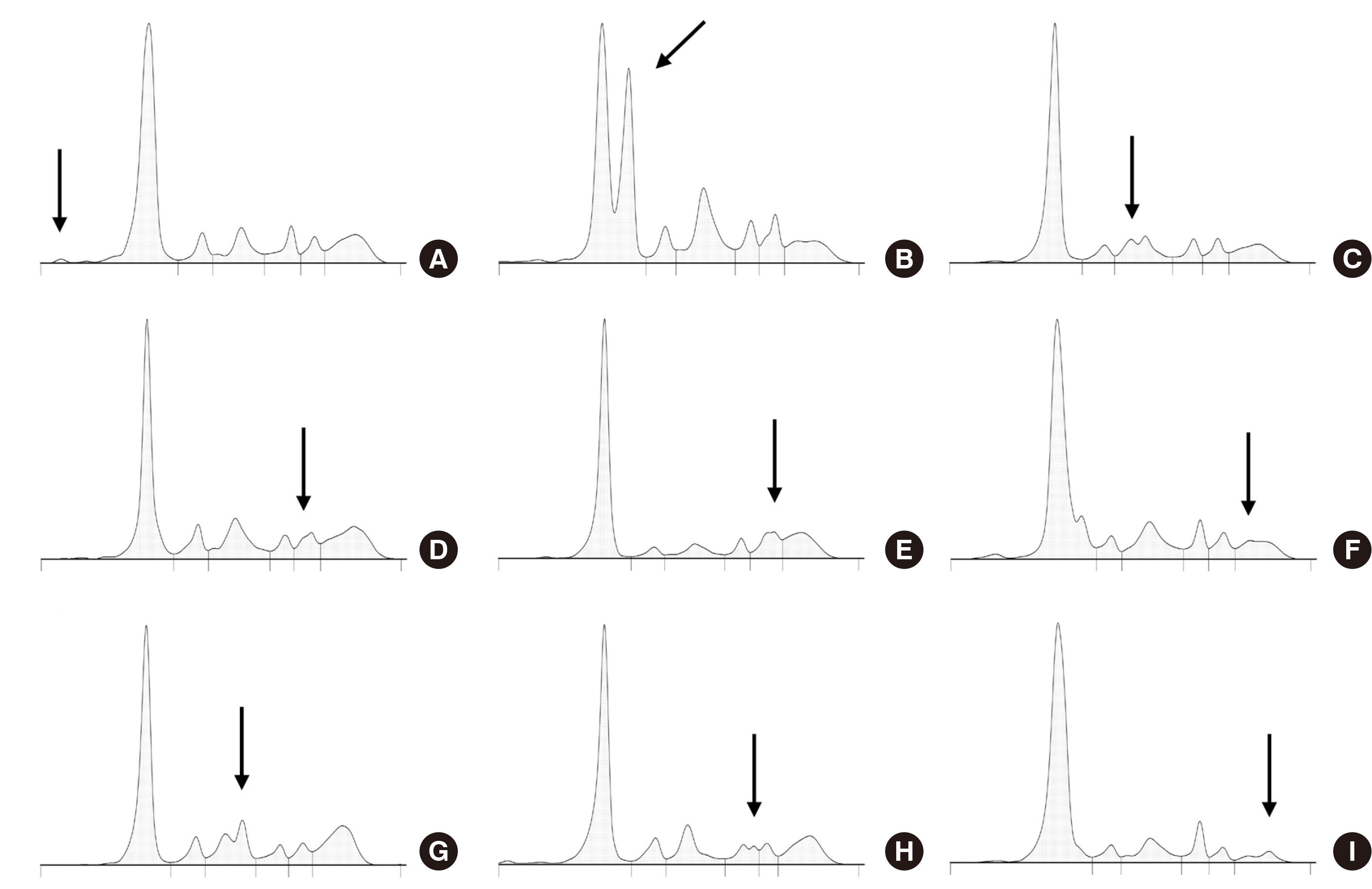
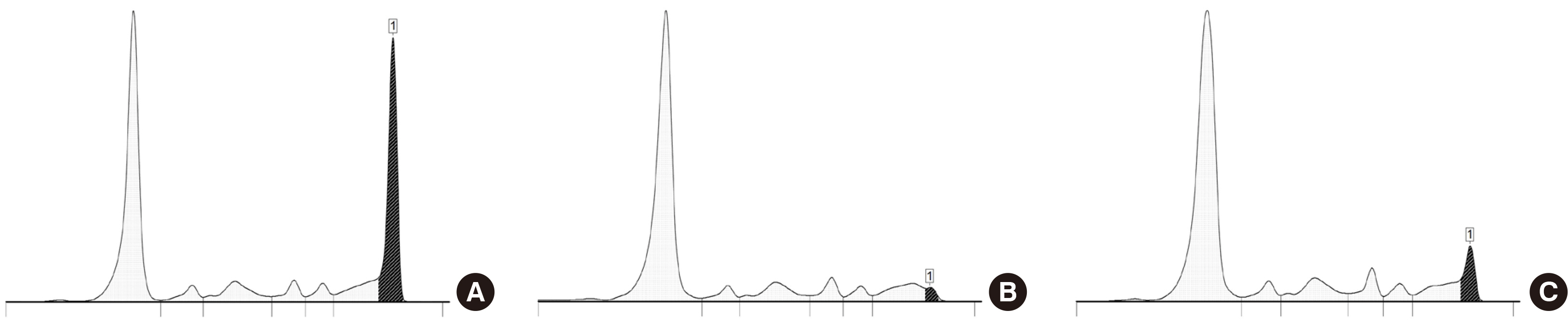
- The use of the serum protein capillary electrophoresis (CE) is prevalent in clinical laboratories owing to its greater feasibility for automation compared with the gel-based methods. Compared with the traditionally commonly used agarose gel electrophoresis, CE exhibits distinctive test principle and technical characteristics, thereby showing different test problems and electrophoretogram characteristics. Proficient comprehension of these properties facilitates the resolution of diverse test problems and verification of results. Electrophoretogram of CE may manifest diverse abnormal peaks beyond monoclonal immunoglobulin protein. Interpreter should understand their characteristics and respond appropriately to abnormal results. To accurately interpret the serum protein CE test, the interpreter would be required to understand the test method and gain experience with various cases through appropriate training.
Keyword
Figure
Reference
-
1. Keren DF. 2012. Protein electrophoresis in clinical diagnosis. American Society for Clinical Pathology Press;Chicago, IL:2. Jacobs JFM, Turner KA, Graziani MS, Frinack JL, Ettore MW, Tate JR, et al. 2020; An international multi-center serum protein electrophoresis accuracy and M-protein isotyping study. Part II: limit of detection and follow-up of patients with small M-proteins. Clin Chem Lab Med. 58:547–59. DOI: 10.1515/cclm-2019-1105. PMID: 31940285.3. Moss MA. 2016; Moving towards harmonized reporting of serum and urine protein electrophoresis. Clin Chem Lab Med. 54:973–9. DOI: 10.1515/cclm-2015-0937. PMID: 26824981.4. Chan PC, Chen Y, Randell EW. Monoclonal Gammopathy Interest Group CSoCC. 2018; On the path to evidence-based reporting of serum protein electrophoresis patterns in the absence of a discernible monoclonal protein - A critical review of literature and practice suggestions. Clin Biochem. 51:29–37. DOI: 10.1016/j.clinbiochem.2017.09.010. PMID: 28916439.5. Bazydio LAL, Landers JP. Rifai N, Chiu RWK, editors. 2023. Electrophoresis. Tietz textbook of laboratory medicine. 7th ed.St. Louis, MO: Elsevier;p. 225–40.6. Hage DS. 2019; An Overview of CE in clinical analysis. Methods Mol Biol. 1972:3–11. DOI: 10.1007/978-1-4939-9213-3_1. PMID: 30847780.7. Bossuyt X. 2006; Advances in serum protein electrophoresis. Adv Clin Chem. 42:43–80. DOI: 10.1016/S0065-2423(06)42002-3. PMID: 17131624.8. Chen FT, Liu CM, Hsieh YZ, Sternberg JC. 1991; Capillary electrophoresis-a new clinical tool. Clin Chem. 37:14–9. DOI: 10.1093/clinchem/37.1.14. PMID: 1988203.9. Genzen JR, Murray DL, Abel G, Meng QH, Baltaro RJ, Rhoads DD, et al. 2018; Screening and diagnosis of monoclonal gammopathies: an international survey of laboratory practice. Arch Pathol Lab Med. 142:507–15. DOI: 10.5858/arpa.2017-0128-CP. PMID: 29266967.10. Cho J, Lee DH, Rim JH, Lee SG, Kim Y, Kim JH. 2022; Current status of serum protein and immunofixation electrophoresis from 29 hospitals in Korea. Lab Med Online. 12:91–9. DOI: 10.47429/lmo.2022.12.2.91.11. Regeniter A, Siede WH. 2018; Peaks and tails: Evaluation of irregularities in capillary serum protein electrophoresis. Clin Biochem. 51:48–55. DOI: 10.1016/j.clinbiochem.2017.09.017. PMID: 28965683.12. Chartier C, Boularan AM, Dupuy AM, Badiou S, Bargnoux AS, Cognot C, et al. 2011; Evaluation of two automated capillary electrophoresis systems for human serum protein analysis. Clin Biochem. 44:1473–9. DOI: 10.1016/j.clinbiochem.2011.05.022. PMID: 21664899.13. Wijnen PA, van Dieijen-Visser MP. 1996; Capillary electrophoresis of serum proteins. Reproducibility, comparison with agarose gel electrophoresis and a review of the literature. Eur J Clin Chem Clin Biochem. 34:535–45. DOI: 10.1515/cclm.1996.34.7.535. PMID: 8864402.14. Gay-Bellile C, Bengoufa D, Houze P, Le Carrer D, Benlakehal M, Bousquet B, et al. 2003; Automated multicapillary electrophoresis for analysis of human serum proteins. Clin Chem. 49:1909–15. DOI: 10.1373/clinchem.2003.017756. PMID: 14578323.15. Tsui AKY, Curran M, Rowe P, Savoy M, Higgins T. 2018; Now you see it, now you don't: a case of covert (invisible) IgM. Clin Biochem. 51:101–2. DOI: 10.1016/j.clinbiochem.2017.06.008. PMID: 28655558.16. Clark R, Katzmann JA, Kyle RA, Fleisher M, Landers JP. 1998; Differential diagnosis of gammopathies by capillary electrophoresis and immunosubtraction: analysis of serum samples problematic by agarose gel electrophoresis. Electrophoresis. 19:2479–84. DOI: 10.1002/elps.1150191421. PMID: 9820971.17. Keren DF, Gulbranson R, Carey JL, Krauss JC. 2001; 2-Mercaptoethanol treatment improves measurement of an IgMkappa M-protein by capillary electrophoresis. Clin Chem. 47:1326–7. DOI: 10.1093/clinchem/47.7.1326. PMID: 11427473.18. Zetterberg H, Nilsson-Ehle H. 2004; False-negative result in the detection of an IgM monoclonal protein by capillary zone electrophoresis. Clin Chem. 50:1878–80. DOI: 10.1373/clinchem.2004.038372. PMID: 15292071.19. Schild C, Egger F, Kaelin-Lang A, Nuoffer JM. 2011; Monoclonal gammopathy missed by capillary zone electrophoresis. Clin Chem Lab Med. 49:1217–9. DOI: 10.1515/CCLM.2011.189. PMID: 21574878.20. Jenkins MA, Guerin MD. 1996; Optimization of serum protein separation by capillary electrophoresis. Clin Chem. 42:1886. DOI: 10.1093/clinchem/42.11.1886. PMID: 8906101.21. Brouwers A, Schiettekatte G, Mariën G, Bossuyt X. 2007; Interference of ceftriaxone on capillary zone electrophoresis. Clin Chim Acta. 376:255–6. DOI: 10.1016/j.cca.2006.08.013. PMID: 16979607.22. Kobayashi S, Okamura N, Kamoi K, Sugita O. 1995; Bisalbumin (fast and slow type) induced by human pancreatic juice. Ann Clin Biochem. 32:63–7. DOI: 10.1177/000456329503200105. PMID: 7762952.23. Ko DH, Chang HE, Song SH, Yoon H, Park KU, Song J. 2011; Identification of compound heterozygous mutation in a Korean patient with Alpha 1-antitrypsin deficiency. Korean J Lab Med. 31:294–7. DOI: 10.3343/kjlm.2011.31.4.294. PMID: 22016686. PMCID: PMC3190011.24. Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. 2003; Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 78:21–33. DOI: 10.4065/78.1.21. PMID: 12528874.25. Kirk RL, Matsumoto H, Katayama K. 1978; Transferrin variants in Korea and Japan. Jinrui Idengaku Zasshi. 23:1–7. DOI: 10.1007/BF01871376. PMID: 671830.26. Gijbels K, De Coster J, Bossuyt X. 2004; Interference by gelatin-based plasma substitutes in capillary zone electrophoresis. Clin Chem. 50:1473–5. DOI: 10.1373/clinchem.2004.033712. PMID: 15277363.27. Bossuyt X. 2004; Interferences in clinical capillary zone electrophoresis of serum proteins. Electrophoresis. 25:1485–7. DOI: 10.1002/elps.200305820. PMID: 15188230.28. Bossuyt X, Lissoir B, Mariën G, Maisin D, Vunckx J, Blanckaert N, et al. 2003; Automated serum protein electrophoresis by Capillarys. Clin Chem Lab Med. 41:704–10. DOI: 10.1515/CCLM.2003.107. PMID: 12812271.29. Chan PC, Chen J. 2015; Value of reflex testing based on hypogammaglobulinemia as demonstrated in serum protein electrophoresis. Clin Biochem. 48:674–8. DOI: 10.1016/j.clinbiochem.2015.03.014. PMID: 25828046.30. Lakshminarayanan R, Li Y, Janatpour K, Beckett L, Jialal I. 2007; Detection by immunofixation of M proteins in hypogammaglobulinemic patients with normal serum protein electrophoresis results. Am J Clin Pathol. 127:746–51. DOI: 10.1309/QJ3PY18PMMJ8AYEH. PMID: 17439833.31. McCudden CR, Jacobs JFM, Keren D, Caillon H, Dejoie T, Andersen K. 2018; Recognition and management of common, rare, and novel serum protein electrophoresis and immunofixation interferences. Clin Biochem. 51:72–9. DOI: 10.1016/j.clinbiochem.2017.08.013. PMID: 28843491.32. Arranz-Peña ML, González-Sagrado M, Olmos-Linares AM, Fernández-García N, Martín-Gil FJ. 2000; Interference of iodinated contrast media in serum capillary zone electrophoresis. Clin Chem. 46:736–7. DOI: 10.1093/clinchem/46.5.736. PMID: 10794768.33. Wheeler RD, Zhang L, Sheldon J. 2018; Large abnormal peak on capillary zone electrophoresis due to contrast agent. Ann Clin Biochem. 55:608–11. DOI: 10.1177/0004563217745896. PMID: 29153027.34. Mills JR, Murray DL. 2017; Identification of friend or foe: the laboratory challenge of differentiating M-proteins from monoclonal antibody therapies. J Appl Lab Med. 1:421–31. DOI: 10.1373/jalm.2016.020784. PMID: 33636806.35. McCudden C, Axel AE, Slaets D, Dejoie T, Clemens PL, Frans S, et al. 2016; Monitoring multiple myeloma patients treated with daratumumab: teasing out monoclonal antibody interference. Clin Chem Lab Med. 54:1095–104. DOI: 10.1515/cclm-2015-1031. PMID: 27028734.36. Noori S, Verkleij CPM, Zajec M, Langerhorst P, Bosman PWC, de Rijke YB, et al. 2021; Monitoring the M-protein of multiple myeloma patients treated with a combination of monoclonal antibodies: the laboratory solution to eliminate interference. Clin Chem Lab Med. 59:1963–71. DOI: 10.1515/cclm-2021-0399. PMID: 34392637.37. Liu L, Shurin MR, Wheeler SE. 2020; A novel approach to remove interference of therapeutic monoclonal antibody with serum protein electrophoresis. Clin Biochem. 75:40–7. DOI: 10.1016/j.clinbiochem.2019.10.011. PMID: 31669513. PMCID: PMC6928417.38. Thoren KL, Pianko MJ, Maakaroun Y, Landgren CO, Ramanathan LV. 2019; Distinguishing drug from disease by use of the Hydrashift 2/4 daratumumab assay. J Appl Lab Med. 3:857–63. DOI: 10.1373/jalm.2018.026476. PMID: 31639760. PMCID: PMC7484995.39. Huls F, Schroeder L, Keren DF. 2020; Expression of daratumumab and elotuzumab migration by capillary electrophoresis relative to transferrin improves precision of their identification. J Appl Lab Med. 5:419–22. DOI: 10.1093/jalm/jfz008. PMID: 32445376.40. Katzmann JA, Clark R, Sanders E, Landers JP, Kyle RA. 1998; Prospective study of serum protein capillary zone electrophoresis and immunotyping of monoclonal proteins by immunosubtraction. Am J Clin Pathol. 110:503–9. DOI: 10.1093/ajcp/110.4.503. PMID: 9763037.41. Bossuyt X, Bogaerts A, Schiettekatte G, Blanckaert N. 1998; Detection and classification of paraproteins by capillary immunofixation/subtraction. Clin Chem. 44:760–4. DOI: 10.1093/clinchem/44.4.760. PMID: 9554486.42. Howard BM, Kuh A, Rezavi L, Caturegli P. 2021; A comparison of gel (Hydragel 30) and capillary (Capillarys III Tera) electrophoresis for the characterization of human serum proteins. Pract Lab Med. 25:e00233. DOI: 10.1016/j.plabm.2021.e00233. PMID: 34095418. PMCID: PMC8145771.43. Poisson J, Fedoriw Y, Henderson MP, Hainsworth S, Tucker K, Uddin Z, et al. 2012; Performance evaluation of the Helena V8 capillary electrophoresis system. Clin Biochem. 45:697–9. DOI: 10.1016/j.clinbiochem.2012.03.018. PMID: 22465274.44. Huang RS, Oleske DA, Tholpady A, Chang BN, Dasgupta A, Nguyen A, et al. 2014; High false-positive rate for monoclonal gammopathy using capillary electrophoresis (CAPILLARYS 2) alone. J Clin Lab Anal. 28:42–6. DOI: 10.1002/jcla.21641. PMID: 24375896. PMCID: PMC6807474.45. Lissoir B, Wallemacq P, Maisin D. 2003; Serum protein electrophoresis: comparison of capillary zone electrophoresis Capillarys (Sebia) and agarose gel electrophoresis Hydrasys (Sebia). Ann Biol Clin (Paris). 61:557–62.46. McCudden CR, Mathews SP, Hainsworth SA, Chapman JF, Hammett-Stabler CA, Willis MS, et al. 2008; Performance comparison of capillary and agarose gel electrophoresis for the identification and characterization of monoclonal immunoglobulins. Am J Clin Pathol. 129:451–8. DOI: 10.1309/6KT8N49BRNVVVBT1. PMID: 18285269.47. Keren DF, Bocsi G, Billman BL, Etzell J, Faix JD, Kumar S, et al. 2022; Laboratory detection and initial diagnosis of monoclonal gammopathies. Arch Pathol Lab Med. 146:575–90. DOI: 10.5858/arpa.2020-0794-CP. PMID: 34347866.48. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. 2014; International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 15:e538–48. DOI: 10.1016/S1470-2045(14)70442-5. PMID: 25439696.49. Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. 2016; International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 17:e328–46. DOI: 10.1016/S1470-2045(16)30206-6. PMID: 27511158.50. Turner KA, Frinack JL, Ettore MW, Tate JR, Graziani MS, Jacobs JFM, et al. 2020; An international multi-center serum protein electrophoresis accuracy and M-protein isotyping study. Part I: factors impacting limit of quantitation of serum protein electrophoresis. Clin Chem Lab Med. 58:533–46. DOI: 10.1515/cclm-2019-1104. PMID: 31940284.51. Tate J, Caldwell G, Daly J, Gillis D, Jenkins M, Jovanovich S, et al. 2012; Recommendations for standardized reporting of protein electrophoresis in Australia and New Zealand. Ann Clin Biochem. 49:242–56. DOI: 10.1258/acb.2011.011158. PMID: 22402916.52. Tate JR, Keren DF, Mollee P. 2018; A global call to arms for clinical laboratories - Harmonised quantification and reporting of monoclonal proteins. Clin Biochem. 51:4–9. DOI: 10.1016/j.clinbiochem.2017.11.009. PMID: 29154955.53. Yang Z, Harrison K, Park YA, Chaffin CH, Thigpen B, Easley PL, et al. 2007; Performance of the Sebia CAPILLARYS 2 for detection and immunotyping of serum monoclonal paraproteins. Am J Clin Pathol. 128:293–9. DOI: 10.1309/1L3CG8GK6F8VYNYH. PMID: 17638665.54. Thoren KL, McCash SI, Murata K. 2021; Immunotyping provides equivalent results to immunofixation in a population with a high prevalence of monoclonal gammopathies. J Appl Lab Med. 6:1551–60. DOI: 10.1093/jalm/jfab067. PMID: 34329441. PMCID: PMC8561783.55. Miyazaki K, Suzuki K. 2016; Capillary electrophoresis/immunosubtraction as a better alternative to immunofixation for detecting and immunotyping serum monoclonal proteins in patients with immunoglobulin light chain (AL) amyloidosis. Amyloid. 23:221–4. DOI: 10.1080/13506129.2016.1232647. PMID: 27682970.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Multiple Myeloma Showing Marked Differences in Serum IgG Levels between Protein Electrophoresis and Turbidimetry
- Evaluation of the Screening Tests for the Diagnosis of Plasma Cell Neoplasm
- SDS-Agarose Gel Electrophoresis Patterns of Urine Protein and Serum Protein Electrophoresis Patterns in the Diabetic Nephropathies
- Study on the Fractionation of Synovial Fluid Protein
- Studies of serieal serum electrophoretic pattern for prognosis in various cancer patients during irradiation