Lab Med Online.
2023 Jul;13(3):230-236. 10.47429/lmo.2023.13.3.230.
An Evaluation of the Appropriateness of Platelet Transfusion and Transfusion Effects of Platelet Products
- Affiliations
-
- 1Department of Laboratory Medicine, Pusan National University Hospital, Busan, Korea
- 2Office of Transfusion Management, Pusan National University Yangsan Hospital, Yangsan, Korea
- 3Department of Laboratory Medicine and Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, Korea
- 4Medical Research Institute, Pusan National University Hospital, Busan, Korea
- 5Department of Laboratory Medicine, Pusan National University School of Medicine, Busan, Korea
- KMID: 2552748
- DOI: http://doi.org/10.47429/lmo.2023.13.3.230
Abstract
- Background
Platelet count is important in assessing patient response after platelet transfusion. Despite their numerous side effects, platelet transfusions lack research on their appropriateness. In this study, the corrected count increment (CCI) 1 hr and 24 hr after platelet transfusion was measured to assess the appropriateness and therapeutic response to platelet transfusion according to blood transfusion guidelines.
Methods
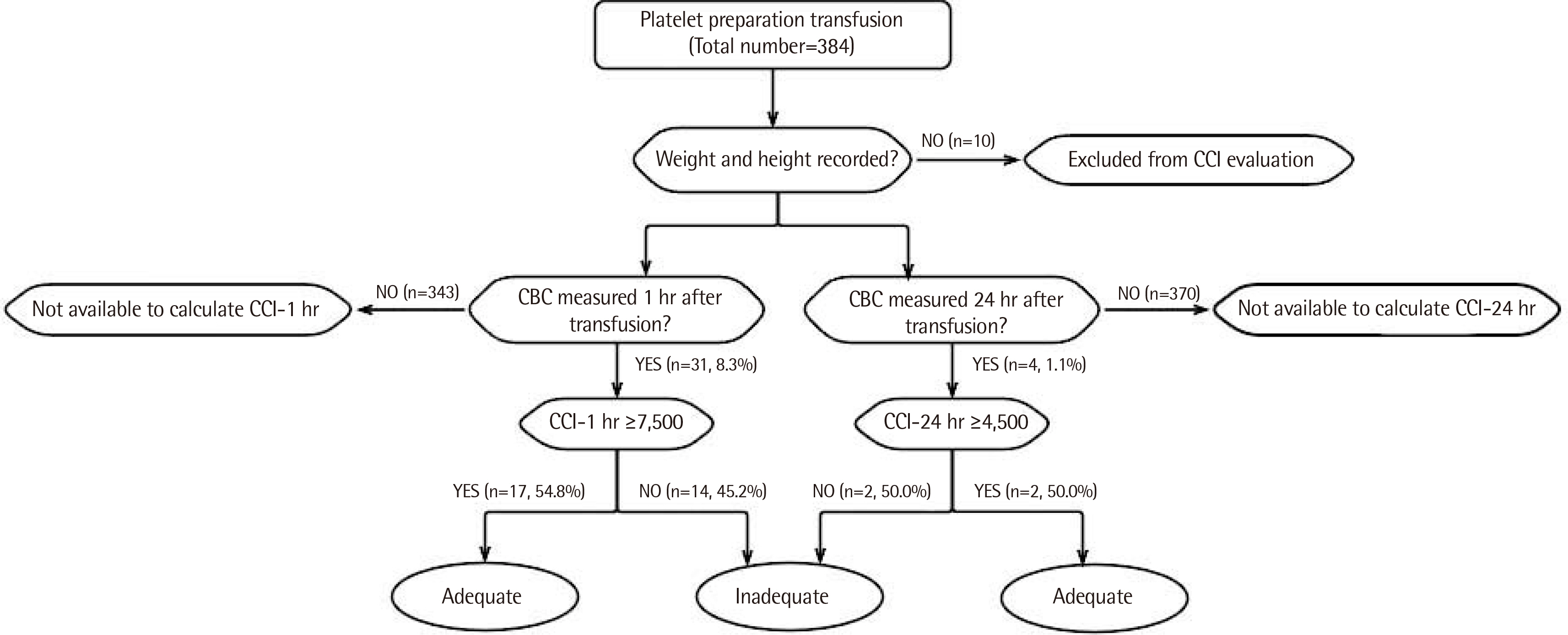
Platelet counts and individual characteristics were reviewed in platelet transfused samples for 9 months. A national transfusion guideline was used to determine platelet transfusion appropriateness in 384 samples, and the therapeutic response to platelet transfusion was evaluated according to CCI at 1 and 24 hr after transfusion in 374 samples, excluding 10 samples from patients who did not have height and weight records.
Results
Platelet transfusions were performed 384 times with 2,729 units of platelet product. A total of 245 (63.8%) platelet transfusions were done under transfusion guidelines. CCI-1 hr and CCI-24 hr could be calculated in 31 (8.3%) and four (1.1%) of 374 samples, with the results of CCI-1 hr ≥ 7,500 and CCI-24 hr ≥ 4,500 in 17 of 31 cases (54.8%) and two of four cases (50.0%), respectively.
Conclusions
It is important to evaluate the adequacy and effectiveness of transfusions using appropriate indicators. HLA-compatible platelet preparation might improve platelet transfusion effectiveness when immunological processes are the suspected causes of platelet incompatibility. Furthermore, since platelet transfusions vary by medical setting, evaluating the appropriateness and therapeutic effects of platelet transfusions at various points within a hospital could be helpful for patient blood management.
Keyword
Figure
Reference
-
1. The Korean Society of Blood Transfusion. Transfusion guidline. 5th ed. 2022.2. Youk HJ, Hwang SH, Oh HB, Ko DH. 2022; Evaluation and management of platelet transfusion refractoriness. Blood Res. 57:6–10. DOI: 10.5045/br.2022.2021229. PMID: 35483919. PMCID: PMC9057673.3. Korean Red Cross, Blood Service Head Quarter. Blood services statistics 2022. https://kosis.kr/statHtml/statHtml.do?orgId=445&tblId=DT_445001_008. Updated on Feb 2023.4. Korean Red Cross, Blood Service Head Quarter. 2021. Blood services statistics.5. Katus MC, Szczepiorkowski ZM, Dumont LJ, Dunbar NM. 2014; Safety of platelet transfusion: past, present and future. Vox Sang. 107:103–13. DOI: 10.1111/vox.12146. PMID: 24650183.6. Han KS, Park KU, editors. 2014. Transfusion Medicine. 4th ed. Korea Medical;Seoul:7. Kiefel V, König C, Kroll H, Santoso S. 2001; Platelet alloantibodies in transfused patients. Transfusion. 41:766–70. DOI: 10.1046/j.1537-2995.2001.41060766.x. PMID: 11399817.8. Korean Red Cross. 2020. Guide to the preparation, use, and quality assurarance of blood component.9. Giudeline for appropriated transfusion, 2020. In. Health Insurance Review & Assessment Service (HIRA), 2020.07.10. Estcourt LJ, Birchall J, Lowe D, Grant-Casey J, Rowley M, Murphy MF. 2012; Platelet transfusions in haematology patients: are we using them appropriately? Vox Sang. 103:284–93. DOI: 10.1111/j.1423-0410.2012.01627.x. PMID: 22775395.11. Hill-Strathy M, Pinkerton PH, Thompson TA, Wendt A, Collins A, Cohen R, et al. 2021; Evaluating the appropriateness of platelet transfusions compared with evidence-based platelet guidelines: an audit of platelet transfusions at 57 hospitals. Transfusion. 61:57–71. DOI: 10.1111/trf.16134. PMID: 33078852.12. Etchells M, Spradbrow J, Cohen R, Lin Y, Armali C, Lieberman L, et al. 2018; Audit of appropriate use of platelet transfusions: validation of adjudication criteria. Vox Sang. 113:40–50. DOI: 10.1111/vox.12550. PMID: 29052231.13. Kim H, Park KU, Han KS. 2010; Blood utilization: audit of transfusion practice using an electronic review system. Korean J Blood Transfus. 21:93–104.14. American Association of Blook Banks. 2008. Standards for blood banks and transfusion services. 25th ed. American Association of Blood Banks;Bethesda, MD:15. Delaflor-Weiss E, Mintz PD. 2000; The evaluation and management of platelet refractoriness and alloimmunization. Transfus Med Rev. 14:180–96. DOI: 10.1016/S0887-7963(00)80007-3. PMID: 10782501.16. Trial to Reduce Alloimmunization to Platelets Study Group. 1997; Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med. 1861–9. DOI: 10.1056/NEJM199712253372601. PMID: 9417523.17. Stanworth SJ, Navarrete C, Estcourt L, Marsh J. 2015; Platelet refractoriness-practical approaches and ongoing dilemmas in patient management. Br J Haematol. 171:297–305. DOI: 10.1111/bjh.13597. PMID: 26194869.18. Hod E, Schwartz J. 2008; Platelet transfusion refractoriness. Br J Haematol. 142:348–60. DOI: 10.1111/j.1365-2141.2008.07189.x. PMID: 18510692.19. Shastry S, Chaudhary R. 2012; Clinical factors influencing corrected count increment. Transfus Apher Sci. 47:327–30. DOI: 10.1016/j.transci.2012.04.006. PMID: 22705296.20. Kim Y, Lim AH, Kim TE, Jung CH, Park M, Seon J, et al. 2021; Current status of Korean Red Cross HLA-matched platelet donor registry. Korean J Blood Transfus. 32:1–10. DOI: 10.17945/kjbt.2021.32.1.1.21. Rebulla P. 2005; A mini-review on platelet refractoriness. Haematologica. 90:247–53.22. Jaime-Pérez JC, Vázquez-Hernández KE, Jiménez-Castillo RA, Fernández LT, Salazar-Riojas R, Gómez-Almaguer D. 2018; Platelet survival in hematology patients assessed by the corrected count increment and other formulas. Am J Clin Pathol. 150:267–72. DOI: 10.1093/ajcp/aqy052. PMID: 29982409.23. Holbro A, Infanti L, Sigle J, Buser A. 2013; Platelet transfusion: basic aspects. Swiss Med Wkly. 143:w13885. DOI: 10.4414/smw.2013.13885. PMID: 24338781.24. Slichter SJ, Davis K, Enright H, Braine H, Gernsheimer T, Kao KJ, et al. 2005; Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood. 105:4106–14. DOI: 10.1182/blood-2003-08-2724. PMID: 15692069. PMCID: PMC1895076.25. Prawita AAAL, Mulyantari NK, Herawati S. The description of corrected count increment on one hour and 24 hours after platelet apheresis transfusion in Sanglah General Hospital Denpasar. Bali Med J. 2019; 8.:445–9. DOI: 10.15562/bmj.v8i2.1391.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reduction of the Platelet Transfusion Dose and Its Effects
- Guidelines for Appropriate and Safe Transfusion
- A Case of Idiopathic Thrombocytopenic Purpura with Anti-E Antibody Developed after Transfusion of Platelet Concentrates
- Evaluation and management of platelet transfusion refractoriness
- Effect of Platelet Transfusion on the Platelet Parameters of Recipient