Lab Med Online.
2023 Jan;13(1):53-59. 10.47429/lmo.2023.13.1.53.
Usefulness of Lymphocyte Subset Analysis Using Primary Immunodeficiency Orientation Tube Panel: Two Cases with Primary Immunodeficiency Diseases
- Affiliations
-
- 1Department of Laboratory Medicine, Pusan National University Hospital, Busan, Korea
- 2Biomedical Research Institute, Pusan National University Hospital, Busan, Korea
- 3Department of Laboratory Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea
- 4Department of Pediatrics, Pusan National University Children’s Hospital, Yangsan, Korea
- KMID: 2552726
- DOI: http://doi.org/10.47429/lmo.2023.13.1.53
Abstract
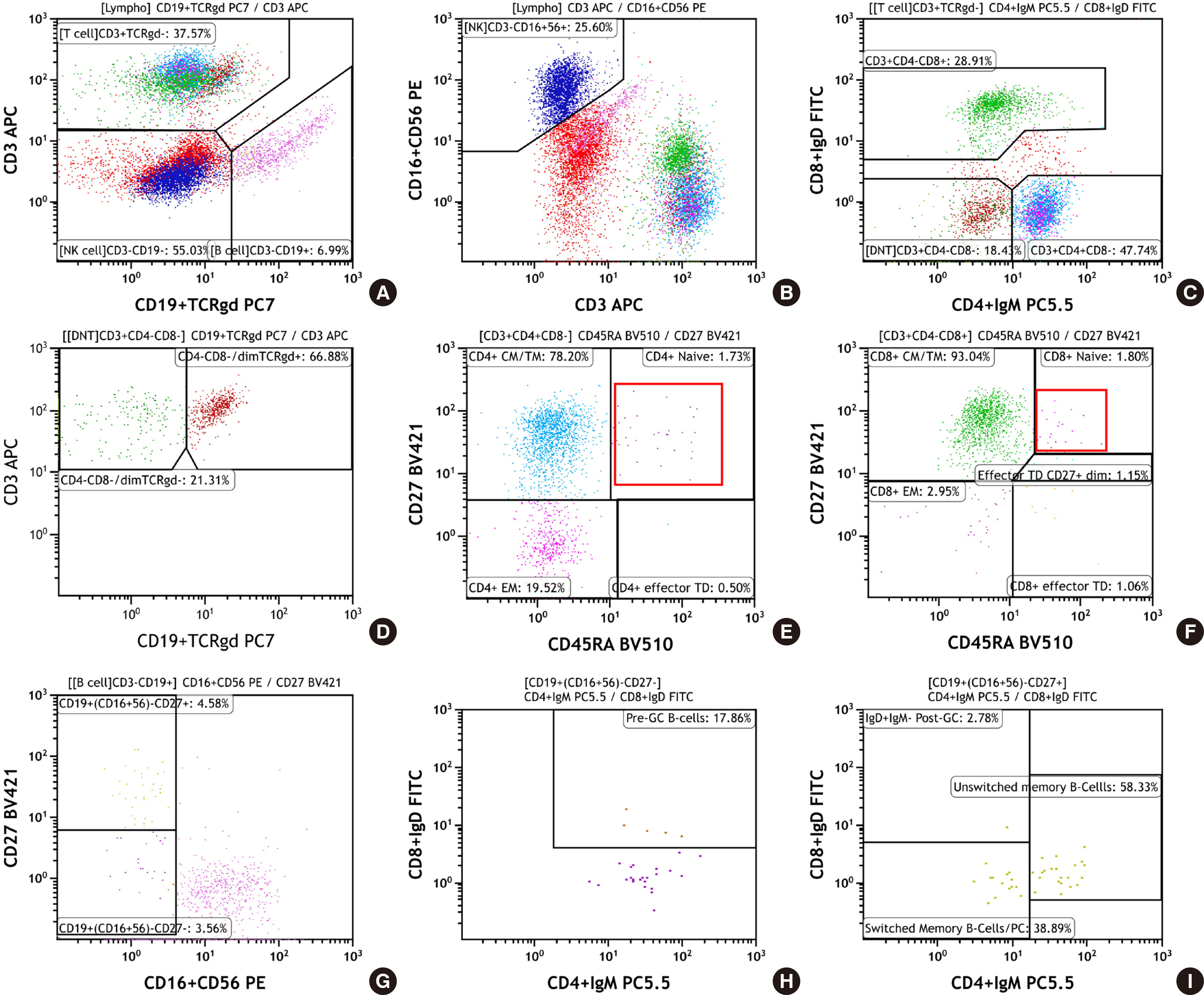
- Primary immunodeficiency diseases (PIDs) are a heterogeneous group of disorders caused by genetic defects in the immune system and exhibit a variety of clinical manifestations, including recurrent infections. Rapid and proper diagnosis is vital for proper therapeutic intervention. Compared to the conventional lymphocyte subset analysis, the standardized Primary Immunodeficiency Orientation Tube (PIDOT) panel has been proposed to provide a more detailed dissection of lymphocyte subsets according to their differentiation and maturation stages. This helps in screening of a suspected PID sample, and to provide quick guidance regarding further course of testing. Here, we report two cases of PID diagnosed by lymphocyte subset analysis using PIDOT panel to establish its usefulness in a clinical laboratory.
Keyword
Figure
Reference
-
1. Tangye SG, Al-Herz W, Bousfiha A, Chatila T, Cunningham-Rundles C, Etzioni A, et al. 2020; Human inborn errors of immunity: 2019 update on the classification from the International Union of Immunological Societies Expert Committee. J Clin Immunol. 40:24–64. DOI: 10.1007/s10875-019-00737-x. PMID: 31953710. PMCID: PMC7082301.2. Abolhassani H, Azizi G, Sharifi L, Yazdani R, Mohsenzadegan M, Delavari S, et al. 2020; Global systematic review of primary immunodeficiency registries. Expert Rev Clin Immunol. 16:717–32. DOI: 10.1080/1744666X.2020.1801422. PMID: 32720819.3. Rhim JW, Kim KH, Kim DS, Kim BS, Kim JS, Kim CH, et al. 2012; Prevalence of primary immunodeficiency in Korea. J Korean Med Sci. 27:788–93. DOI: 10.3346/jkms.2012.27.7.788. PMID: 22787376. PMCID: PMC3390729.4. Leiding JW, Forbes LR. 2019; Mechanism-based precision therapy for the treatment of primary immunodeficiency and primary immunodysregulatory diseases. J Allergy Clin Immunol Pract. 7:761–73. DOI: 10.1016/j.jaip.2018.12.017. PMID: 30832891.5. Aguilar C, Malphettes M, Donadieu J, Chandesris O, Coignard-Biehler H, Catherinot E, et al. 2014; Prevention of infections during primary immunodeficiency. Clin Infect Dis. 59:1462–70. DOI: 10.1093/cid/ciu646. PMID: 25124061.6. Long PM, Sanford KW, Bluth MH. McPherson RA, Pincus MR, editors. 2017. Immunodeficiency disorders. Henry's clinical diagnosis and management by laboratory methods. 23rd ed. p. 984–92. St. Louis, MO: Elsevier.7. Abraham RS, Aubert G. 2016; Flow cytometry, a versatile tool for diagnosis and monitoring of primary immunodeficiencies. Clin Vaccine Immunol. 23:254–71. DOI: 10.1128/CVI.00001-16. PMID: 26912782. PMCID: PMC4820507.8. van der Burg M, Kalina T, Perez-Andres M, Vlkova M, Lopez-Granados E, Blanco E, et al. 2019; The EuroFlow PID Orientation Tube for flow cytometric dDiagnostic screening of primary immunodeficiencies of the lymphoid system. Front Immunol. 10:246. DOI: 10.3389/fimmu.2019.00246. PMID: 30886612. PMCID: PMC6410673.9. Ding Y, Zhou L, Xia Y, Wang W, Wang Y, Li L, et al. 2018; Reference values for peripheral blood lymphocyte subsets of healthy children in China. J Allergy Clin Immunol. 142:970–3. DOI: 10.1016/j.jaci.2018.04.022. PMID: 29746882.10. Warnatz K, Denz A, Dräger R, Braun M, Groth C, Wolff-Vorbeck G, et al. 2002; Severe deficiency of switched memory B cells (CD27(+)IgM(-)IgD(-)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 99:1544–51. DOI: 10.1182/blood.V99.5.1544. PMID: 11861266.11. Del Pino-Molina L, López-Granados E, Lecrevisse Q, Torres Canizales J, Pérez-Andrés M, Blanco E, et al. 2021; Dissection of the pre-germinal center B-cell maturation pathway in common variable immunodeficiency based on standardized fFlow cytometric EuroFlow tools. Front Immunol. 11:603972. DOI: 10.3389/fimmu.2020.603972. PMID: 33679693. PMCID: PMC7925888.12. Delmonte OM, Schuetz C, Notarangelo LD. 2018; RAG deficiency: two genes, many Diseases. J Clin Immunol. 38:646–55. DOI: 10.1007/s10875-018-0537-4. PMID: 30046960. PMCID: PMC6643099.13. Kalina T, Bakardjieva M, Blom M, Perez-Andres M, Barendregt B, Kanderová V, et al. 2020; EuroFlow standardized approach to diagnostic immunopheneotyping of severe PID in newborns and young children. Front Immunol. 11:371. DOI: 10.3389/fimmu.2020.00371. PMID: 32265901. PMCID: PMC7096355.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection and Diagnosis of Primary Immunodeficiency Diseases
- Clinical Features of Primary Immunodeficiency Diseases
- A Case of Ankylosing Spondylitis in a Patient with Human Immunodeficiency Virus
- Genetic diagnosis of systemic autoinflammatory diseases and underlying primary immunodeficiency
- A Clinical Study of Primary Immunodeficiency Disease in a Single Center in Seoul from 1996 to 2004