Ann Lab Med.
2023 Nov;43(6):620-624. 10.3343/alm.2023.43.6.620.
Validation of High-sensitivity Flow Cytometry for Reliable Immune Cell Analysis in Real-world Laboratory Settings
- Affiliations
-
- 1Department of Laboratory Medicine, Seoul National University Bundang Hospital and Seoul National University College of Medicine, Seoul, Korea
- 2Department of Surgery, Division of Kidney and Pancreas Transplantation, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2552040
- DOI: http://doi.org/10.3343/alm.2023.43.6.620
Abstract
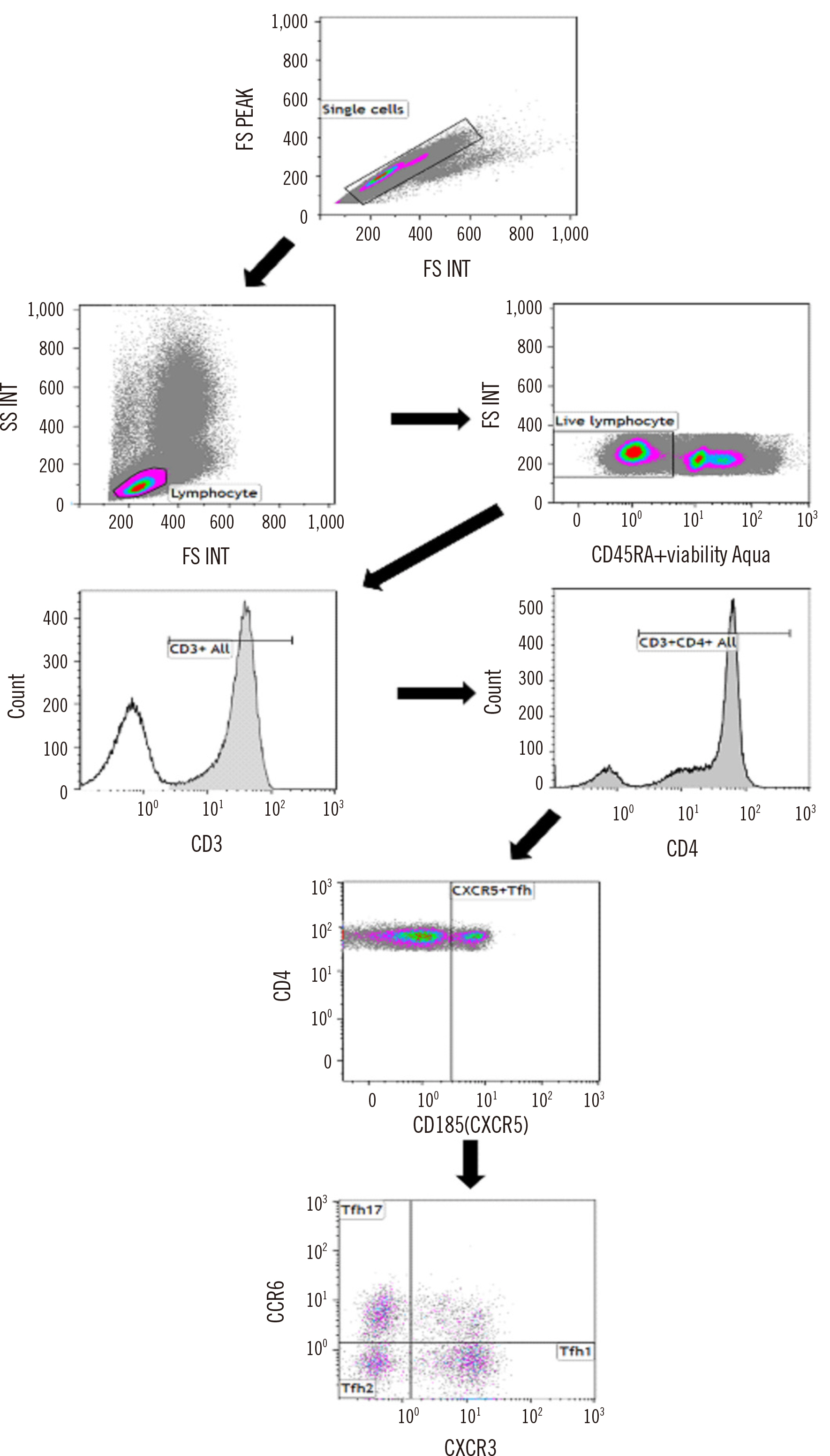
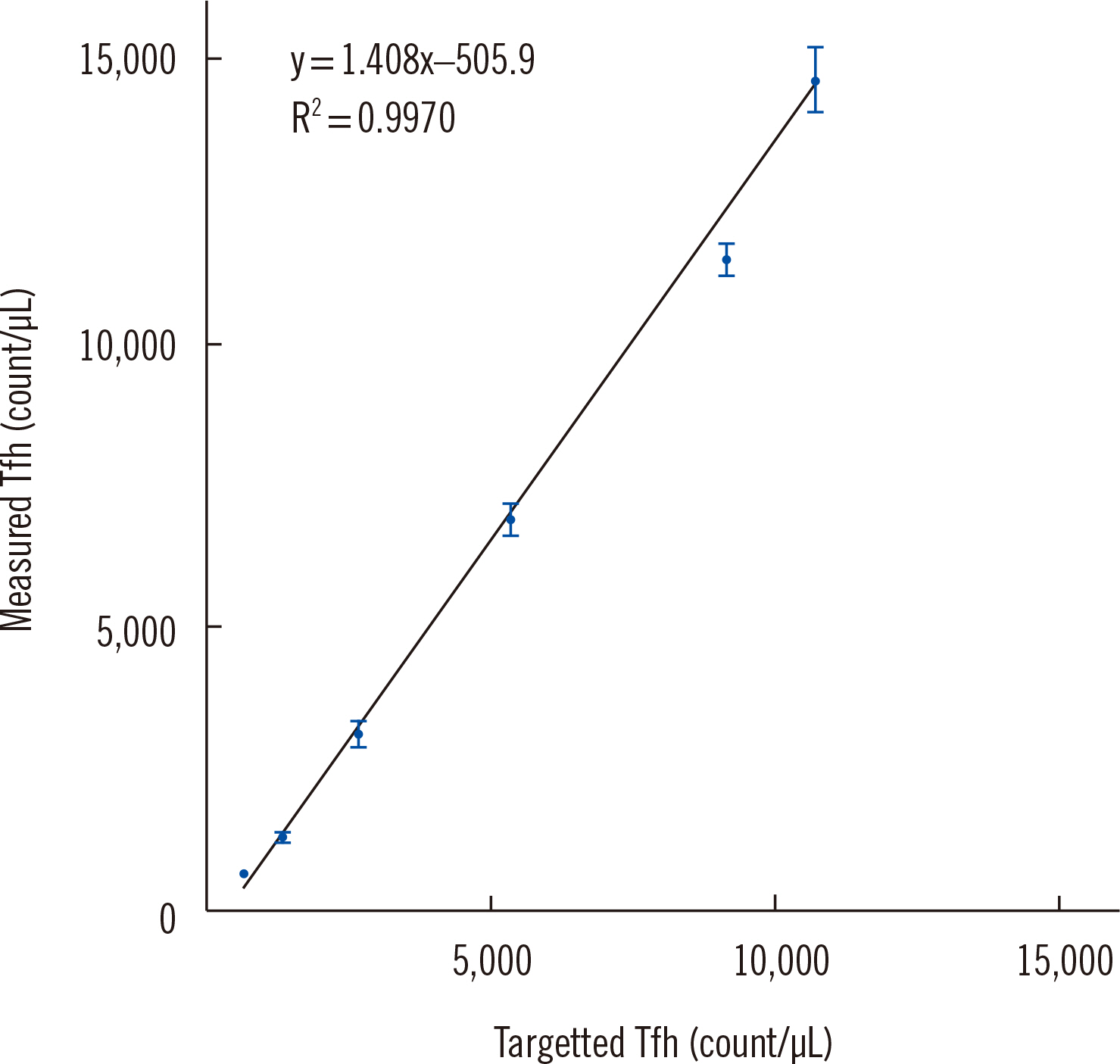
- The adoption of high-sensitivity flow cytometry (HSFC) in routine laboratory settings has been slow owing to concerns regarding the reliability and reproducibility of results. Validation is an essential prerequisite for conducting assays, and implementing the CLSI guidelines has been confusing, primarily because many aspects are not yet established. We aimed to validate an HSFC protocol for detecting follicular helper T (Tfh) cells in a real-world laboratory environment. The analytical validity of the Tfh cell panel was ensured through rigorous testing, including evaluations of precision, stability, carryover, and sensitivity, following the CLSI H62 guidelines. We found that Tfh cells, present in very small numbers in the blood, could be sufficiently detected through HSFC, and concerns about the reliability and reproducibility of the results in real-world laboratories could be solved through systematic validation. Establishing the lower limit of quantification (LLOQ) is a critical step in HSFC evaluations. By selecting an appropriate sample, for example, collecting residual cells from CD4 isolation in our experiment and using them as low-level samples, the LLOQ could be accurately established. The strategic validation of flow cytometry panels can facilitate the adoption of HSFC in clinical laboratories, even with limited resources.
Figure
Reference
-
1. Sommer U, Eck S, Marszalek L, Stewart JJ, Bradford J, McCloskey TW, et al. High‐sensitivity flow cytometric assays: considerations for design control and analytical validation for identification of rare events. Cytometry Part B: Clinical Cytometry. 2021; 100:42–51. DOI: 10.1002/cyto.b.21949. PMID: 32940947.
Article2. Kim HY, Yoo IY, Lim DJ, Kim HJ, Kim SH, Yoon SE, et al. 2022; Clinical utility of next-generation flow-based minimal residual disease assessment in patients with multiple myeloma. Annals of Laboratory Medicine. 42:558–65. DOI: 10.3343/alm.2022.42.5.558. PMID: 35470273. PMCID: PMC9057816.
Article3. Wei Y, Feng J, Hou Z, Wang XM, Yu D. Flow cytometric analysis of circulating follicular helper T (Tfh) and follicular regulatory T (Tfr) populations in human blood. T Follicular Helper Cells: Methods and Protocols. 2015; 199–207. DOI: 10.1007/978-1-4939-2498-1_17. PMID: 25836313.
Article4. Edner NM, Heuts F, Thomas N, Wang CJ, Petersone L, Kenefeck R, et al. 2020; Follicular helper T cell profiles predict response to costimulation blockade in type 1 diabetes. Nature Immunology. 21:1244–55. DOI: 10.1038/s41590-020-0744-z. PMID: 32747817. PMCID: PMC7610476.
Article5. Boswell KL, Paris R, Boritz E, Ambrozak D, Yamamoto T, Darko S, et al. 2014; Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathogens. 10:e1003853. DOI: 10.1371/journal.ppat.1003853. PMID: 24497824. PMCID: PMC3911819.
Article6. Vinuesa CG, Linterman MA, Yu D, MacLennan IC. 2016; Follicular helper T cells. Annual Review of Immunology. 34:335–68. DOI: 10.1146/annurev-immunol-041015-055605. PMID: 26907215.
Article7. Danger R, Chesneau M, Delbos F, Le Bot S, Kerleau C, Chenouard A, et al. 2019; CXCR5+ PD1+ ICOS+ circulating T follicular helpers are associated with de novo donor-specific antibodies after renal transplantation. Frontiers in Immunology. 10:2071. DOI: 10.3389/fimmu.2019.02071. PMID: 31552030. PMCID: PMC6746839.8. Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. 2011; Human blood CXCR5+ CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 34:108–21. DOI: 10.1016/j.immuni.2010.12.012. PMID: 21215658. PMCID: PMC3046815.
Article9. Degandt S, Peeters B, Jughmans S, Boeckx N, Bossuyt X. 2018; Analytical performance of an automated volumetric flow cytometer for quantitation of T, B and natural killer lymphocytes. Clinical Chemistry and Laboratory Medicine (CCLM). 56:1277–88. DOI: 10.1515/cclm-2017-0638. PMID: 29466232.
Article10. Selliah N, Eck S, Green C, Oldaker T, Stewart J, Vitaliti A, et al. 2019; Flow cytometry method validation protocols. Current Protocols in Cytometry. 87:e53. DOI: 10.1002/cpcy.53. PMID: 30418706.
Article11. Schmitt N, Bentebibel SE, Ueno H. 2014; Phenotype and functions of memory Tfh cells in human blood. Trends in Immunology. 35:436–42. DOI: 10.1016/j.it.2014.06.002. PMID: 24998903. PMCID: PMC4152409.
Article12. CLSI. Validation of assays performed by flow cytometry. Laboratory Standards Institute, 2021.13. Hale JS, Ahmed R. 2015; Memory T follicular helper CD4 T cells. Frontiers in Immunology. 6:16. DOI: 10.3389/fimmu.2015.00016. PMID: 25699040. PMCID: PMC4313784.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of T Cells Using Flow Cytometry
- Multiparameter Flow Cytometry: Advances in High Resolution Analysis
- Diagnostic Value of Flow Cytometric DNA Analysis in the Evaluation of Effusions
- The role of flow cytometry in urine cytology
- A Comparative Study of DNA Quantitation by Image Cytometry and Flow Cytometry