Int J Stem Cells.
2024 Feb;17(1):70-79. 10.15283/ijsc22209.
Integrin α 4 Positive Subpopulation in Adipose Derived Stem Cells Effectively Reduces Infarct Size through Enhanced Engraftment into Myocardial Infarction
- Affiliations
-
- 1Department of Vascular Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Hubei Key Laboratory of Biological Targeted Therapy, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 3Medical College, Wuhan University of Science and Technology, Wuhan, China
- KMID: 2552019
- DOI: http://doi.org/10.15283/ijsc22209
Abstract
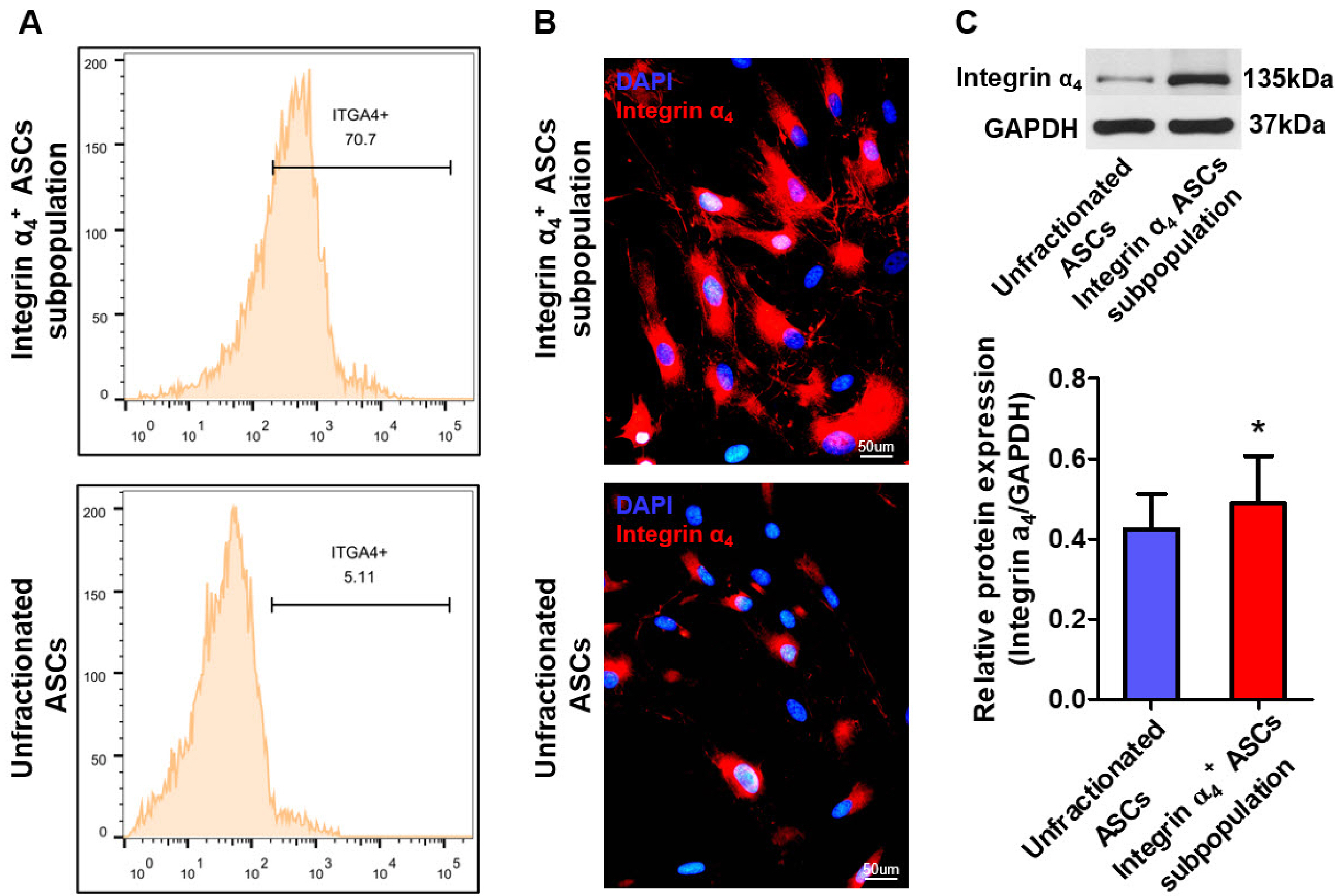
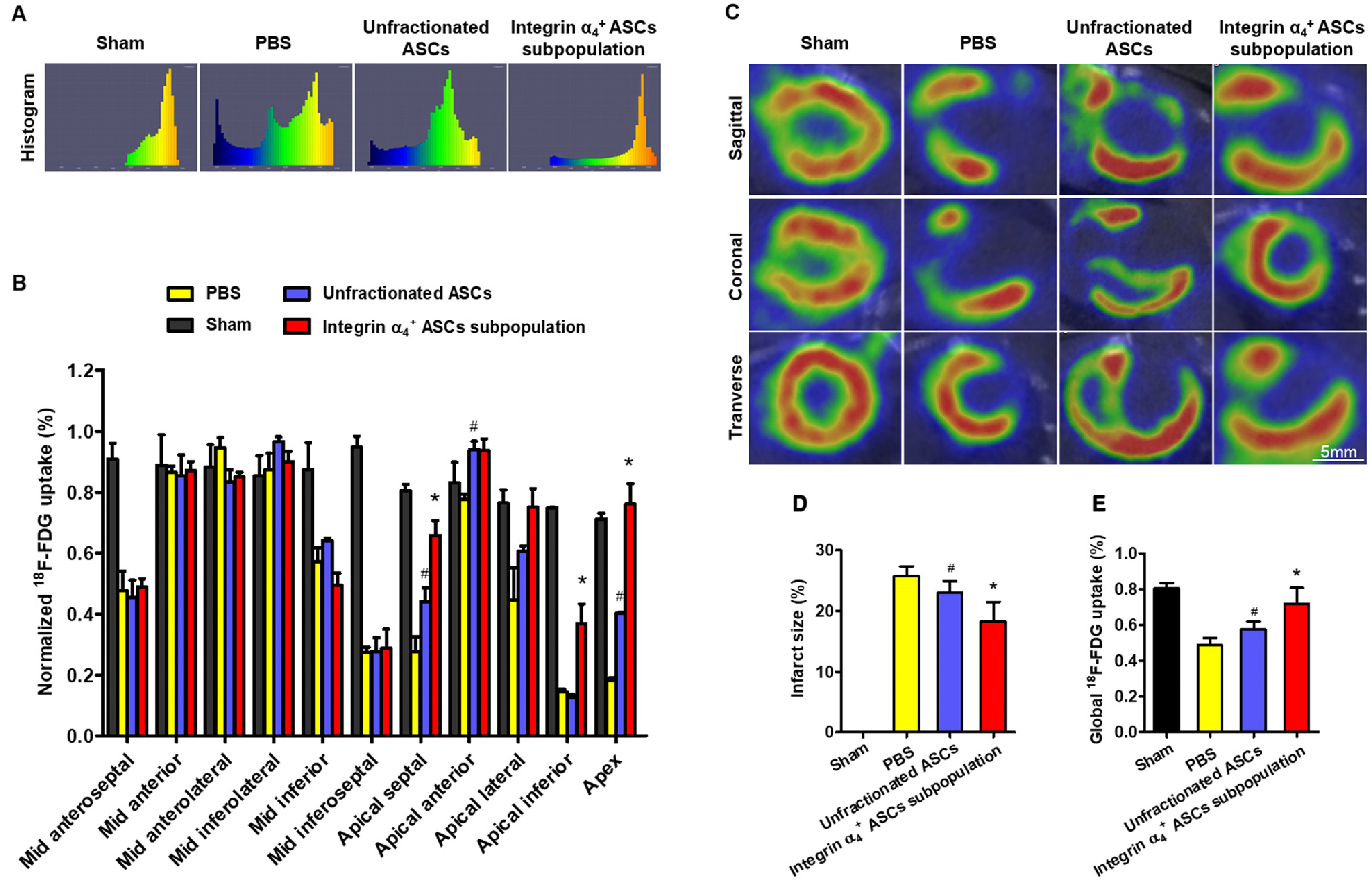
- The efficacy of adipose-derived stem cells (ASCs) on myocardial infarction is limited due to poor survival and engraftment. Integrin-mediated cell adhesion is a prerequisite for its survival and homing. ASCs expressed insufficient integrin α 4 , limiting their homing capacity. This study aims to characterize integrin α 4+ ASC subpopulation and investigate their therapeutic efficacy in myocardial infarction. We used fluorescence-activated cell sorting to harvest integrin α 4+ ASCs subpopulation, which were characterized in vitro and transplanted into myocardial infarction model. Positron emission tomography imaging were performed to measure infarction size. Cardiac cine magnetic resonance imaging was used to evaluate heart contractile function. Compared with the unfractionated ASCs, integrin α 4+ ASCs subpopulation secreted a higher level of angiogenic growth factors, migrated more rapidly, and exhibited a stronger anti-apoptotic capacity. Vascular cell adhesion molecule-1 was obviously up-regulated at 3 days after myocardial infarction, which interacted with integrin α 4 receptor on the surface of ASCs to enhance the survival and adhesion. Thus, we implanted unfractionated ASCs or integrin α 4 Integrin α 4+ ASCs subpopulation exhibited more robust engraftment into the infarcted myocardium. Integrin α 4 ASCs subpopulation more effectively decreased infarct size and strengthen cardiac function recovery than did the unfractionated ASCs. Integrin α 4 ocardial damage in animal model. Mechanistically, their more robust engraftment into the infarct area, higher migratory capacity and their increased release of paracrine factors contribute to enhanced tissue repair.
Keyword
Figure
Reference
-
References
1. Velagaleti RS, Pencina MJ, Murabito JM, et al. 2008; Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 118:2057–2062. DOI: 10.1161/CIRCULATIONAHA.108.784215. PMID: 18955667. PMCID: PMC2729712.
Article2. Planat-Benard V, Silvestre JS, Cousin B, et al. 2004; Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 109:656–663. DOI: 10.1161/01.CIR.0000114522.38265.61. PMID: 14734516.
Article3. Rehman J, Traktuev D, Li J, et al. 2004; Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 109:1292–1298. DOI: 10.1161/01.CIR.0000121425.42966.F1. PMID: 14993122.
Article4. Wang J, Xiang B, Deng JX, et al. 2017; Hypoxia enhances the therapeutic potential of superparamagnetic iron oxide-labeled adipose-derived stem cells for myocardial infarction. J Huazhong Univ Sci Technolog Med Sci. 37:516–522. DOI: 10.1007/s11596-017-1766-0. PMID: 28786062.
Article5. Bai X, Yan Y, Song YH, et al. 2010; Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur Heart J. 31:489–501. DOI: 10.1093/eurheartj/ehp568. PMID: 20037143.
Article6. Wang J, Xiang B, Deng J, et al. 2016; Externally applied static magnetic field enhances cardiac retention and functional benefit of magnetically iron-labeled adipose-derived stem cells in infarcted hearts. Stem Cells Transl Med. 5:1380–1393. DOI: 10.5966/sctm.2015-0220. PMID: 27400797. PMCID: PMC5031175.
Article7. Zhang H, Chen H, Wang W, Wei Y, Hu S. 2010; Cell survival and redistribution after transplantation into damaged myo-cardium. J Cell Mol Med. 14:1078–1082. DOI: 10.1111/j.1582-4934.2010.01076.x. PMID: 20646127. PMCID: PMC3822744.
Article8. Berman AE, Kozlova NI, Morozevich GE. 2003; Integrins: structure and signaling. Biochemistry (Mosc). 68:1284–1299. DOI: 10.1023/B:BIRY.0000011649.03634.74. PMID: 14756624.
Article9. Suna S, Sakata Y, Sato H, et al. 2008; Up-regulation of cell adhesion molecule genes in human endothelial cells stimulated by lymphotoxin alpha: DNA microarray analysis. J Athero-scler Thromb. 15:160–165. DOI: 10.5551/jat.E553. PMID: 18603823.
Article10. Duan H, Cheng L, Sun X, et al. 2006; LFA-1 and VLA-4 involved in human high proliferative potential-endothelial progenitor cells homing to ischemic tissue. Thromb Haemost. 96:807–815. Erratum in: Thromb Haemost 2007;97:167. DOI: 10.1160/TH06-04-0199. PMID: 17139377.
Article11. Song SW, Chang W, Song BW, et al. 2009; Integrin-linked kinase is required in hypoxic mesenchymal stem cells for strengthening cell adhesion to ischemic myocardium. Stem Cells. 27:1358–1365. DOI: 10.1002/stem.47. PMID: 19489098.
Article12. Vitillo L, Baxter M, Iskender B, Whiting P, Kimber SJ. 2016; Integrin-associated focal adhesion kinase protects human embryonic stem cells from apoptosis, detachment, and dif-ferentiation. Stem Cell Reports. 7:167–176. DOI: 10.1016/j.stemcr.2016.07.006. PMID: 27509133. PMCID: PMC4983079. PMID: 117f06df4ec54e0d9683d97667986669.
Article13. Park GC, Kim HS, Park HY, et al. 2019; Tensin-3 regulates integrin-mediated proliferation and differentiation of tonsil-derived mesenchymal stem cells. Cells. 9:89. DOI: 10.3390/cells9010089. PMID: 31905841. PMCID: PMC7017379. PMID: 3caea375ed114661a2d598030baac171.
Article14. Wang H, Li J, Zhang X, et al. 2018; Priming integrin alpha 5 promotes the osteogenic differentiation of human periodontal ligament stem cells due to cytoskeleton and cell cycle changes. J Proteomics. 179:122–130. DOI: 10.1016/j.jprot.2018.03.008. PMID: 29545170.
Article15. Wilschut KJ, van Tol HT, Arkesteijn GJ, Haagsman HP, Roelen BA. 2011; Alpha 6 integrin is important for myogenic stem cell differentiation. Stem Cell Res. 7:112–123. DOI: 10.1016/j.scr.2011.05.001. PMID: 21763619.
Article16. Uitterdijk A, Groenendijk BCW, Gorsse-Bakker C, et al. 2017; Time course of VCAM-1 expression in reperfused myocardial infarction in swine and its relation to retention of intracoronary administered bone marrow-derived mononuclear cells. PLoS One. 12:e0178779. DOI: 10.1371/journal.pone.0178779. PMID: 28628621. PMCID: PMC5476248. PMID: a83bba91e2974dffba77af5086c3d7df.
Article17. Li L, Guan Q, Dai S, Wei W, Zhang Y. 2017; Integrin β1 increases stem cell survival and cardiac function after myocardial infarction. Front Pharmacol. 8:135. DOI: 10.3389/fphar.2017.00135. PMID: 28367125. PMCID: PMC5355448.
Article18. Goto K, Takemura G, Takahashi T, et al. 2016; Intravenous administration of endothelial colony-forming cells overexpre-ssing integrin β1 augments angiogenesis in ischemic legs. Stem Cells Transl Med. 5:218–226. DOI: 10.5966/sctm.2015-0096. PMID: 26702126. PMCID: PMC4729550.
Article19. Cui LL, Nitzsche F, Pryazhnikov E, et al. 2017; Integrin α4 overexpression on rat mesenchymal stem cells enhances transmigration and reduces cerebral embolism after intracarotid injection. Stroke. 48:2895–2900. DOI: 10.1161/STROKEAHA.117.017809. PMID: 28916665.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of miRNA-324-5p-Modified Adipose-Derived Stem Cells in Post-Myocardial Infarction Repair
- Adipose Tissue-Derived Stem Cells for Myocardial Regeneration
- Delivery of SAV-siRNA via Exosomes from Adipose-Derived Stem Cells for the Treatment of Myocardial Infarction
- Stem Cells for Cardiovascular Disease
- Familial Occurrence of Pulmonary Embolism after Intravenous, Adipose Tissue-Derived Stem Cell Therapy