Ann Lab Med.
2023 May;43(3):290-294. 10.3343/alm.2023.43.3.290.
Evaluation of Cellular Responses to ChAdOx1-nCoV-19 and BNT162b2 Vaccinations
- Affiliations
-
- 1Department of Laboratory Medicine, Konkuk University School of Medicine, Seoul, Korea
- 2Department of Laboratory Medicine, Korea University Anam Hospital, Seoul, Korea
- 3Department of Internal Medicine, Konkuk University School of Medicine, Seoul, Korea
- KMID: 2551713
- DOI: http://doi.org/10.3343/alm.2023.43.3.290
Abstract
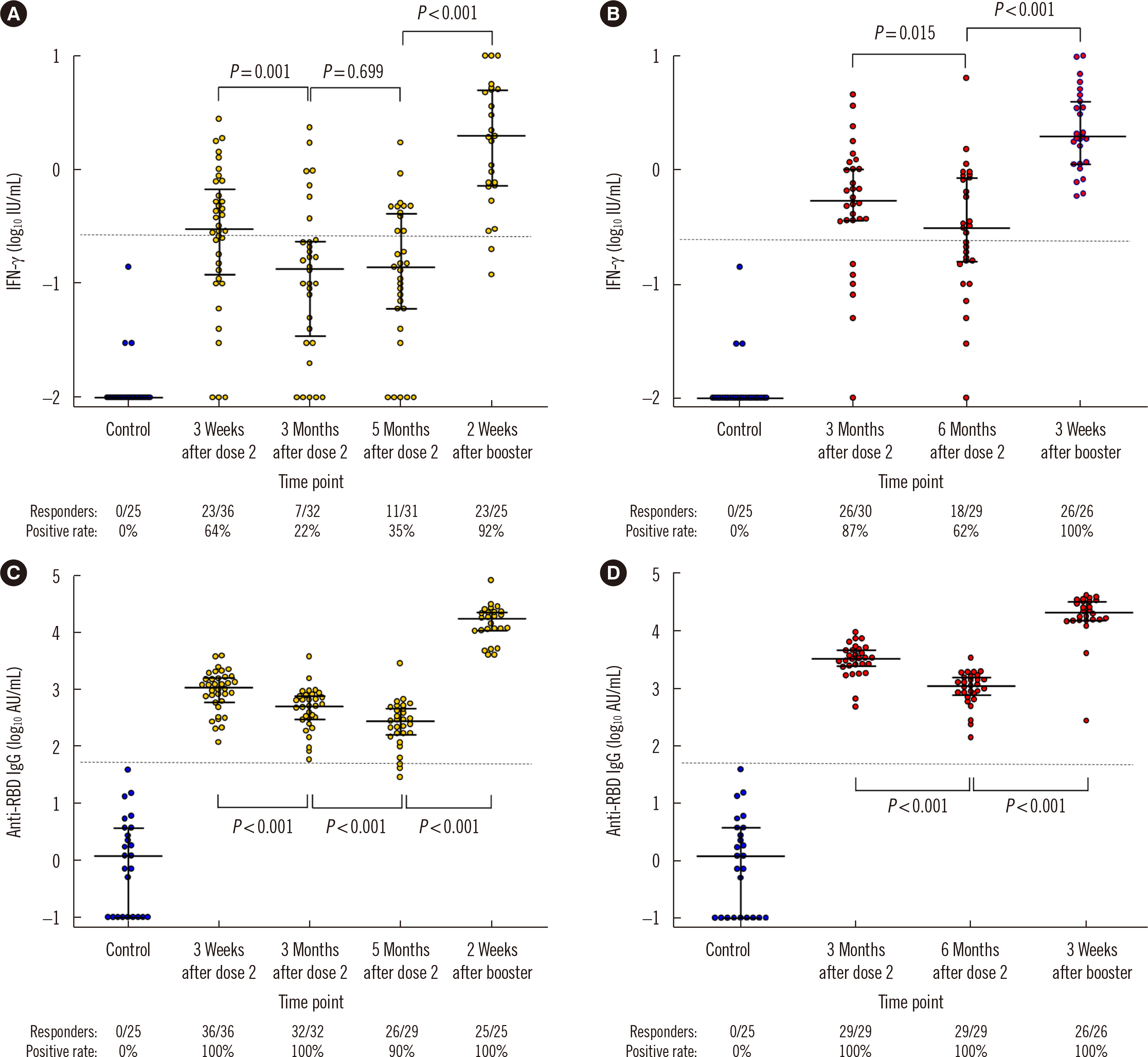
- While numerous studies have evaluated humoral responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines, data on the cellular responses to these vaccines remain sparse. We evaluated T cell responses to ChAdOx1-nCoV-19 and BNT162b2 vaccinations using an interferon gamma (IFN-γ) release assay (IGRA). ChAdOx1-nCoV-19- and BNT162b2-vaccinated participants initially showed stronger T cell responses than unvaccinated controls. The T cell response decreased over time and increased substantially after the administration of a BNT162b2 booster dose. Changes in the T cell response were less significant than those in the anti-receptor-binding domain IgG antibody titer. The study results can serve as baseline data for T cell responses after SARS-CoV-2 vaccination and suggest that the IGRA can be useful in monitoring immunogenicity.
Keyword
Figure
Reference
-
1. Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. 2020; A new coronavirus associated with human respiratory disease in China. Nature. 579:265–9. DOI: 10.1038/s41586-020-2008-3. PMID: 32015508. PMCID: PMC7094943.
Article2. Cham J, Pandey AC, New J, Huynh T, Hong L, Orendain N, et al. 2022; 6 month serologic response to the Pfizer-BioNTech COVID-19 vaccine among healthcare workers. PLoS One. 17:e0266781.
Article3. Robertson LJ, Price R, Moore JS, Curry G, Farnan J, Black A, et al. 2022; IgG antibody production and persistence to 6 months following SARS-CoV-2 vaccination: a Northern Ireland observational study. Vaccine. 40:2535–9. DOI: 10.1016/j.vaccine.2022.02.087. PMID: 35346536. PMCID: PMC8900637.
Article4. Kim JA, Bang HI, Shin JW, Park Y, Kim S, Kim MY, et al. 2022; Immunogenicity of third-dose BNT162b2 mRNA vaccine following two doses of ChAdOx1 in health care workers: a prospective longitudinal study. Ann Lab Med. 42:688–92. DOI: 10.3343/alm.2022.42.6.688. PMID: 35765878. PMCID: PMC9277035.
Article5. Cameroni E, Bowen JE, Rosen LE, Saliba C, Zepeda SK, Culap K, et al. 2022; Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 602:664–70. DOI: 10.1038/s41586-021-04386-2. PMID: 35016195.
Article6. Zhuang Z, Lai X, Sun J, Chen Z, Zhang Z, Dai J, et al. 2021; Mapping and role of T cell response in SARS-CoV-2-infected mice. J Exp Med. 218:e20202187. DOI: 10.1084/jem.2020218710052021c. PMID: 34653240. PMCID: PMC8526302.
Article7. McMahan K, Yu J, Mercado NB, Loos C, Tostanoski LH, Chandrashekar A, et al. 2021; Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 590:630–4. DOI: 10.1038/s41586-020-03041-6. PMID: 33276369. PMCID: PMC7906955.
Article8. Woldemeskel BA, Garliss CC, Blankson JN. 2021; SARS-CoV-2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS-CoV-2 variants and HCoV-NL63. J Clin Invest. 131:e149335. DOI: 10.1172/JCI149335. PMID: 33822770. PMCID: PMC8121504.
Article9. Geers D, Shamier MC, Bogers S, den Hartog G, Gommers L, Nieuwkoop NN, et al. 2021; SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol. 6:eabj1750. DOI: 10.1126/sciimmunol.abj1750. PMID: 34035118. PMCID: PMC9268159.
Article10. Jung MK, Jeong SD, Noh JY, Kim DU, Jung S, Song JY, et al. 2022; BNT162b2-induced memory T cells respond to the Omicron variant with preserved polyfunctionality. Nat Microbiol. 7:909–17. DOI: 10.1038/s41564-022-01123-x. PMID: 35577972.
Article11. Mukaka MM. 2012; Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 24:69–71.12. Busà R, Sorrentino MC, Russelli G, Amico G, Miceli V, Miele M, et al. 2022; Specific anti-SARS-CoV-2 humoral and cellular immune responses after booster dose of BNT162b2 Pfizer-BioNTech mRNA-based vaccine: integrated study of adaptive immune system components. Front Immunol. 13:856657. DOI: 10.3389/fimmu.2022.856657. PMID: 35401503. PMCID: PMC8987231.
Article13. Groß R, Zanoni M, Seidel A, Conzelmann C, Gilg A, Krnavek D, et al. 2022; Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity against prevalent SARS-CoV-2 variants. EBioMedicine. 75:103761. DOI: 10.1016/j.ebiom.2021.103761. PMID: 34929493. PMCID: PMC8682749.
Article14. Shaw RH, Liu X, Stuart ASV, Greenland M, Aley PK, Andrews NJ, et al. Effect of priming interval on reactogenicity, peak immunological response, and waning after homologous and heterologous COVID-19 vaccine schedules: exploratory analyses of Com-COV, a randomised control trial. Lancet Respir Med. 2022; S2213-2600(22)00163-1.
Article15. Qui M, Le Bert N, Chan WPW, Tan M, Hang SK, Hariharaputran S, et al. 2022; Favorable vaccine-induced SARS-CoV-2-specific T cell response profile in patients undergoing immune-modifying therapies. J Clin Invest. 132:e159500. DOI: 10.1172/JCI159500. PMID: 35536644. PMCID: PMC9197512.
Article16. Yao L, Wang GL, Shen Y, Wang ZY, Zhan BD, Duan LJ, et al. 2021; Persistence of antibody and cellular immune responses in coronavirus disease 2019 patients over nine months after infection. J Infect Dis. 224:586–94. DOI: 10.1093/infdis/jiab255. PMID: 33978754. PMCID: PMC8243600.
Article17. Le Bert N, Tan AT, Kunasegaran K, Tham CYL, Hafezi M, Chia A, et al. 2020; SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 584:457–62. DOI: 10.1038/s41586-020-2550-z. PMID: 32668444.
Article18. Chen J, Wang R, Gilby NB, Wei GW. 2022; Omicron variant (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model. 62:412–22. DOI: 10.1021/acs.jcim.1c01451. PMID: 34989238. PMCID: PMC8751645.
Article19. Tarke A, Coelho CH, Zhang Z, Dan JM, Yu ED, Methot N, et al. 2022; SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell. 185:847–59.e11. DOI: 10.1016/j.cell.2022.01.015. PMID: 35139340. PMCID: PMC8784649.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Antibody and T Cell Responses Induced by Single Doses of ChAdOx1 nCoV-19 and BNT162b2 Vaccines
- Adverse Events in Healthcare Workers after the First Dose of ChAdOx1 nCoV-19 or BNT162b2 mRNA COVID-19 Vaccination: a Single Center Experience
- Comparison of antibody responses after the 1st and 2nd doses of COVID-19 vaccine with those of patients with mild or severe COVID-19
- Two Case Reports of Leukocytoclastic Vasculitis after ChAdOx1 nCoV-19 Vaccine
- Comparison of humoral immunogenicity in solid organ transplant recipients after third-dose mRNA vaccine with homologous or heterologous schedules: an observational study