Intest Res.
2024 Jan;22(1):8-14. 10.5217/ir.2023.00087.
Precision medicine in inflammatory bowel diseases
- Affiliations
-
- 1Division of Gastroenterology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
- KMID: 2551276
- DOI: http://doi.org/10.5217/ir.2023.00087
Abstract
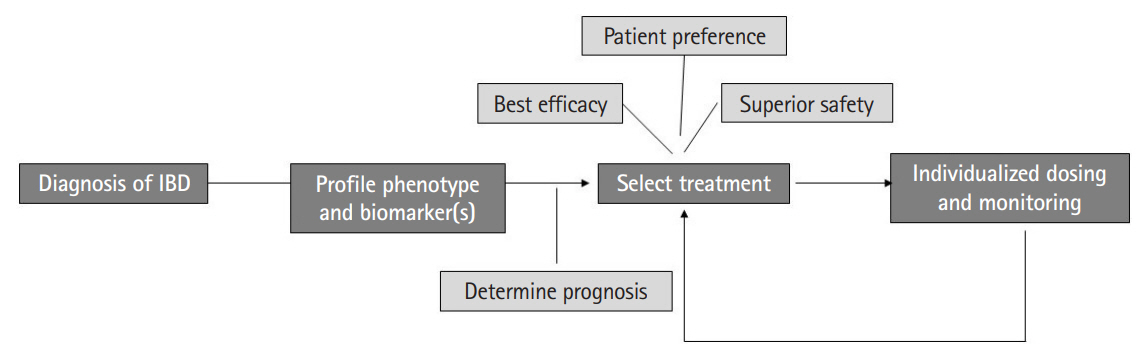
- Inflammatory bowel diseases comprising Crohn’s disease and ulcerative colitis have emerged as global diseases. Multiple distinct therapeutic mechanisms have allowed us to increase our rates of achieving remission and reducing permanent disease-related morbidity. However, there is limited data to inform relative positioning of different therapies. This review will summarize existing literature on use of clinical decision models to inform relative efficacy of one therapeutic mechanism compared to the other given individual patient characteristics. It will also demonstrate the value of serologic, transcriptomic (from biopsies), and microbiome-based biomarkers in identifying which therapy is most likely to work for a given patient. We will review the existing gaps in the literature in this field and suggest a path forward for future studies to better inform patient care, incorporating the principles of precision medicine in the management of inflammatory bowel disease.
Figure
Reference
-
1. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017; 390:2769–2778.
Article2. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017; 389:1756–1770.
Article3. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet. 2017; 389:1741–1755.
Article4. Jefremow A, Neurath MF. Novel small molecules in IBD: current state and future perspectives. Cells. 2023; 12:1730.
Article5. Alsoud D, Verstockt B, Fiocchi C, Vermeire S. Breaking the therapeutic ceiling in drug development in ulcerative colitis. Lancet Gastroenterol Hepatol. 2021; 6:589–595.
Article6. Torres J, Caprioli F, Katsanos KH, et al. Predicting outcomes to optimize disease management in inflammatory bowel diseases. J Crohns Colitis. 2016; 10:1385–1394.
Article7. Kobayashi T, Hisamatsu T, Suzuki Y, et al. Predicting outcomes to optimize disease management in inflammatory bowel disease in Japan: their differences and similarities to Western countries. Intest Res. 2018; 16:168–177.
Article8. Siegel CA, Horton H, Siegel LS, et al. A validated web-based tool to display individualised Crohn’s disease predicted outcomes based on clinical, serologic and genetic variables. Aliment Pharmacol Ther. 2016; 43:262–271.
Article9. Livanos AE, Dunn A, Fischer J, et al. Anti-integrin αvβ6 autoantibodies are a novel biomarker that antedate ulcerative colitis. Gastroenterology. 2023; 164:619–629.10. Biasci D, Lee JC, Noor NM, et al. A blood-based prognostic biomarker in IBD. Gut. 2019; 68:1386–1395.
Article11. Privitera G, Pugliese D, Rapaccini GL, Gasbarrini A, Armuzzi A, Guidi L. Predictors and early markers of response to biological therapies in inflammatory bowel diseases. J Clin Med. 2021; 10:853.
Article12. Siegel CA, Melmed GY. Predicting response to anti-TNF agents for the treatment of Crohn’s disease. Therap Adv Gastroenterol. 2009; 2:245–251.
Article13. Sands BE, Sandborn WJ, Van Assche G, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease in patients naïve to or who have failed tumor necrosis factor antagonist therapy. Inflamm Bowel Dis. 2017; 23:97–106.
Article14. Nagano K, Hata E, Asano T, et al. Safety and effectiveness of ustekinumab for Crohn’s disease in Japanese Post-marketing Surveillance in biologic-naive and -experienced conriemed. Crohns Colitis 360. 2023; 5–otad001.
Article15. Vande Casteele N, Jairath V, Jeyarajah J, et al. Development and validation of a clinical decision support tool that incorporates pharmacokinetic data to predict endoscopic healing in patients treated with infliximab. Clin Gastroenterol Hepatol. 2021; 19:1209–1217.
Article16. Dulai PS, Boland BS, Singh S, et al. Development and validation of a scoring system to predict outcomes of vedolizumab treatment in patients with Crohn’s disease. Gastroenterology. 2018; 155:687–695.
Article17. Dulai PS, Singh S, Vande Casteele N, et al. Development and validation of clinical scoring tool to predict outcomes of treatment with vedolizumab in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2020; 18:2952–2961.
Article18. Dulai PS, Wong EC, Reinisch W, Colombel JF, Marshall JK, Narula N. Decision support tool identifies ulcerative colitis patients most likely to achieve remission with vedolizumab vs adalimumab. Inflamm Bowel Dis. 2022; 28:1555–1564.
Article19. Park J, Chun J, Yoon H, Cheon JH. Feasibility of a clinical decision support tool for ustekinumab to predict clinical remission and relapse in patients with Crohn’s disease: a multicenter observational study. Inflamm Bowel Dis. 2023; 29:548–554.
Article20. Narula N, Wong EC, Dulai PS, Marshall JK, Jairath V, Reinisch W. Comparative effectiveness of biologics for endoscopic healing of the ileum and colon in Crohn’s disease. Am J Gastroenterol. 2022; 117:1106–1117.
Article21. Singh S, Heien HC, Herrin J, et al. Comparative risk of serious infections with tumor necrosis factor α antagonists vs vedolizumab in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2022; 20:e74–e88.
Article22. Kirchgesner J, Desai RJ, Beaugerie L, Schneeweiss S, Kim SC. Risk of serious infections with vedolizumab versus tumor necrosis factor antagonists in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2022; 20:314–324.
Article23. Cheng D, Kochar B, Cai T, Ritchie CS, Ananthakrishnan AN. Comorbidity influences the comparative safety of biologic therapy in older adults with inflammatory bowel diseases. Am J Gastroenterol. 2022; 117:1845–1850.
Article24. Barber GE, Yajnik V, Khalili H, et al. Genetic markers predict primary non-response and durable response to anti-TNF biologic therapies in Crohn’s disease. Am J Gastroenterol. 2016; 111:1816–1822.
Article25. Billiet T, Papamichael K, de Bruyn M, et al. A matrix-based model predicts primary response to infliximab in Crohn’s disease. J Crohns Colitis. 2015; 9:1120–1126.
Article26. Arijs I, Li K, Toedter G, et al. Mucosal gene signatures to predict response to infliximab in patients with ulcerative colitis. Gut. 2009; 58:1612–1619.
Article27. Arijs I, Quintens R, Van Lommel L, et al. Predictive value of epithelial gene expression profiles for response to infliximab in Crohn’s disease. Inflamm Bowel Dis. 2010; 16:2090–2098.
Article28. West NR, Hegazy AN, Owens BM, et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med. 2017; 23:579–589.29. Verstockt B, Verstockt S, Dehairs J, et al. Low TREM1 expression in whole blood predicts anti-TNF response in inflammatory bowel disease. EBioMedicine. 2019; 40:733–742.
Article30. Verstockt B, Verstockt S, Veny M, et al. Expression levels of 4 genes in colon tissue might be used to predict which patients will enter endoscopic remission after vedolizumab therapy for inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2020; 18:1142–1151.
Article31. Martin JC, Chang C, Boschetti G, et al. Single-cell analysis of Crohn’s disease lesions identifies a pathogenic cellular module associated with resistance to anti-TNF therapy. Cell. 2019; 178:1493–1508.
Article32. Smillie CS, Biton M, Ordovas-Montanes J, et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell. 2019; 178:714–730.
Article33. Kolho KL, Korpela K, Jaakkola T, et al. Fecal microbiota in pediatric inflammatory bowel disease and its relation to inflammation. Am J Gastroenterol. 2015; 110:921–930.
Article34. Sanchis-Artero L, Martínez-Blanch JF, Manresa-Vera S, et al. Evaluation of changes in intestinal microbiota in Crohn’s disease patients after anti-TNF alpha treatment. Sci Rep. 2021; 11:10016.
Article35. Zhou Y, Xu ZZ, He Y, et al. Gut microbiota offers universal biomarkers across ethnicity in inflammatory bowel disease diagnosis and infliximab response prediction. mSystems. 2018; 3:e00188–17.
Article36. Ananthakrishnan AN, Luo C, Yajnik V, et al. Gut Microbiome function predicts response to anti-integrin biologic therapy in inflammatory bowel diseases. Cell Host Microbe. 2017; 21:603–610.
Article37. Lee JW, Plichta D, Hogstrom L, et al. Multi-omics reveal microbial determinants impacting responses to biologic therapies in inflammatory bowel disease. Cell Host Microbe. 2021; 29:1294–1304.
Article38. Sazonovs A, Kennedy NA, Moutsianas L, et al. HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with Crohn’s disease. Gastroenterology. 2020; 158:189–199.39. Walker GJ, Harrison JW, Heap GA, et al. Association of genetic variants in NUDT15 with thiopurine-induced myelosuppression in patients with inflammatory bowel disease. JAMA. 2019; 321:773–785.40. Heap GA, Weedon MN, Bewshea CM, et al. HLA-DQA1-HLADRB1 variants confer susceptibility to pancreatitis induced by thiopurine immunosuppressants. Nat Genet. 2014; 46:1131–1134.
Article41. Dubinsky MC, Mendiolaza ML, Phan BL, Moran HR, Tse SS, Mould DR. Dashboard-driven accelerated infliximab induction dosing increases infliximab durability and reduces immunogenicity. Inflamm Bowel Dis. 2022; 28:1375–1385.
Article42. Strik AS, Löwenberg M, Mould DR, et al. Efficacy of dashboard driven dosing of infliximab in inflammatory bowel disease patients; a randomized controlled trial. Scand J Gastroenterol. 2021; 56:145–154.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Natural Product-Derived Drugs for the Treatment of Inflammatory Bowel Diseases
- Can vitamin D supplementation help control inflammation in inflammatory bowel disease beyond its classical role in bone health?
- Precision medicine for pediatric inflammatory bowel disease: a perspective
- Animal Model for Inflammatory Bowel Disease
- Monitoring and Safety of Azathioprine Therapy in Inflammatory Bowel Disease