Clin Endosc.
2024 Jan;57(1):24-35. 10.5946/ce.2023.036.
Use of artificial intelligence in the management of T1 colorectal cancer: a new tool in the arsenal or is deep learning out of its depth?
- Affiliations
-
- 1Department of Gastroenterology and Hepatology, Changi General Hospital, Singapore Health Services, Singapore
- 2Academic Medicine Center, Duke-NUS Medical School, Singapore
- 3Department of Laboratory Medicine, Changi General Hospital, Singapore Health Services, Singapore
- 4Digestive Disease Center, Showa University Northern Yokohama Hospital, Yokohama, Japan
- 5Yong Loo Lin School of Medicine, National University of Singapore, Singapore
- 6Department of General Surgery, Changi General Hospital, Singapore Health Services, Singapore
- KMID: 2551187
- DOI: http://doi.org/10.5946/ce.2023.036
Abstract
- The field of artificial intelligence is rapidly evolving, and there has been an interest in its use to predict the risk of lymph node metastasis in T1 colorectal cancer. Accurately predicting lymph node invasion may result in fewer patients undergoing unnecessary surgeries; conversely, inadequate assessments will result in suboptimal oncological outcomes. This narrative review aims to summarize the current literature on deep learning for predicting the probability of lymph node metastasis in T1 colorectal cancer, highlighting areas of potential application and barriers that may limit its generalizability and clinical utility.
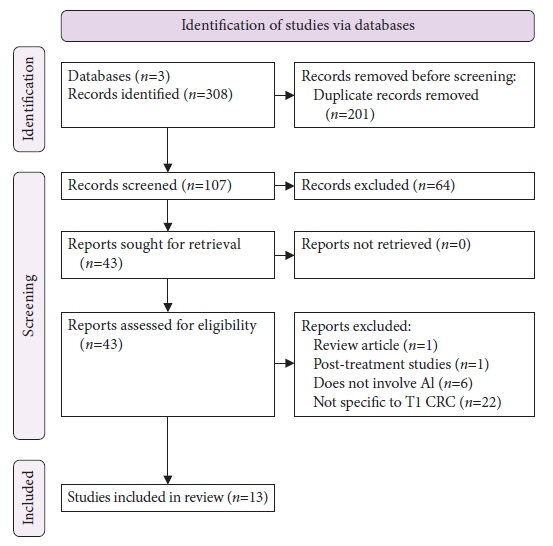
Figure
Reference
-
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–249.2. Xi Y, Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl Oncol. 2021; 14:101174.3. Bretthauer M, Kaminski MF, Løberg M, et al. Population-based colonoscopy screening for colorectal cancer: a randomized clinical trial. JAMA Intern Med. 2016; 176:894–902.4. Dekker E, Tanis PJ, Vleugels JL, et al. Colorectal cancer. Lancet. 2019; 394:1467–1480.5. Draganov PV, Wang AY, Othman MO, et al. AGA Institute Clinical Practice Update: endoscopic submucosal dissection in the United States. Clin Gastroenterol Hepatol. 2019; 17:16–25.6. Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015; 47:829–854.7. Dykstra MA, Gimon TI, Ronksley PE, et al. Classic and novel histopathologic risk factors for lymph node metastasis in T1 colorectal cancer: a systematic review and meta-analysis. Dis Colon Rectum. 2021; 64:1139–1150.8. Niimi K, Fujishiro M, Kodashima S, et al. Long-term outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy. 2010; 42:723–729.9. Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc. 2010; 72:1217–1225.10. Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020; 25:1–42.11. Japanese Society for Cancer of the Colon and Rectum. Japanese classification of colorectal, appendiceal, and anal carcinoma: the 3d English edition [secondary publication]. J Anus Rectum Colon. 2019; 3:175–195.12. Tateishi Y, Nakanishi Y, Taniguchi H, et al. Pathological prognostic factors predicting lymph node metastasis in submucosal invasive (T1) colorectal carcinoma. Mod Pathol. 2010; 23:1068–1072.13. Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017; 28(suppl_4):iv22–iv40.14. Benson AB, Venook AP, Al-Hawary MM, et al. Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018; 16:874–901.15. Bosch SL, Teerenstra S, de Wilt JH, et al. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy. 2013; 45:827–834.16. Ebbehøj AL, Jørgensen LN, Krarup PM, et al. Histopathological risk factors for lymph node metastases in T1 colorectal cancer: meta-analysis. Br J Surg. 2021; 108:769–776.17. Nascimbeni R, Burgart LJ, Nivatvongs S, et al. Risk of lymph node metastasis in T1 carcinoma of the colon and rectum. Dis Colon Rectum. 2002; 45:200–206.18. Yamamoto S, Watanabe M, Hasegawa H, et al. The risk of lymph node metastasis in T1 colorectal carcinoma. Hepatogastroenterology. 2004; 51:998–1000.19. Zong Z, Li H, Hu CG, et al. Predictors of lymph-node metastasis in surgically resected T1 colorectal cancer in Western populations. Gastroenterol Rep (Oxf). 2021; 9:470–474.20. Vermeer NC, Backes Y, Snijders HS, et al. National cohort study on postoperative risks after surgery for submucosal invasive colorectal cancer. BJS Open. 2018; 3:210–217.21. Ichimasa K, Kudo SE, Miyachi H, et al. Current problems and perspectives of pathological risk factors for lymph node metastasis in T1 colorectal cancer: systematic review. Dig Endosc. 2022; 34:901–912.22. Hassan C, Spadaccini M, Iannone A, et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 2021; 93:77–85.23. Sivananthan A, Nazarian S, Ayaru L, et al. Does computer-aided diagnostic endoscopy improve the detection of commonly missed polyps?: a meta-analysis. Clin Endosc. 2022; 55:355–364.24. Li JW, Chia T, Fock KM, et al. Artificial intelligence and polyp detection in colonoscopy: use of a single neural network to achieve rapid polyp localization for clinical use. J Gastroenterol Hepatol. 2021; 36:3298–3307.25. Li JW, Ang TL. Colonoscopy and artificial intelligence: bridging the gap or a gap needing to be bridged? Artif Intell Gastrointest Endosc. 2021; 2:36–49.26. Chen H, Sung JJ. Potentials of AI in medical image analysis in gastroenterology and hepatology. J Gastroenterol Hepatol. 2021; 36:31–38.27. Collins GS, Moons KG. Reporting of artificial intelligence prediction models. Lancet. 2019; 393:1577–1579.28. CONSORT-AI and SPIRIT-AI Steering Group. Reporting guidelines for clinical trials evaluating artificial intelligence interventions are needed. Nat Med. 2019; 25:1467–1468.29. Nagendran M, Chen Y, Lovejoy CA, et al. Artificial intelligence versus clinicians: systematic review of design, reporting standards, and claims of deep learning studies. BMJ. 2020; 368:m689.30. Rivera SC, Liu X, Chan AW, et al. Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI Extension. BMJ. 2020; 370:m3210.31. Ahmad OF, Mori Y, Misawa M, et al. Establishing key research questions for the implementation of artificial intelligence in colonoscopy: a modified Delphi method. Endoscopy. 2021; 53:893–901.32. Li JW, Ang TL. Narrow-band imaging. In : Chiu PW, Sano Y, Uedo N, Singh R, editors. Endoscopy in early gastrointestinal cancers, volume 1: diagnosis. Singapore: Springer Singapore;2021. p. 111–119.33. Sano Y, Tanaka S, Kudo SE, et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc. 2016; 28:526–533.34. Ito R, Ikematsu H, Murano T, et al. Diagnostic ability of Japan Narrow-Band Imaging Expert Team classification for colorectal lesions by magnifying endoscopy with blue laser imaging versus narrow-band imaging. Endosc Int Open. 2021; 9:E271–E277.35. Hewett DG, Kaltenbach T, Sano Y, et al. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012; 143:599–607.36. Kaltenbach T, Anderson JC, Burke CA, et al. Endoscopic removal of colorectal lesions-recommendations by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2020; 158:1095–1129.37. Desai M, Kennedy K, Aihara H, et al. External validation of blue light imaging (BLI) criteria for the optical characterization of colorectal polyps by endoscopy experts. J Gastroenterol Hepatol. 2021; 36:2728–2734.38. Bisschops R, East JE, Hassan C, et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) guideline: update 2019. Endoscopy. 2019; 51:1155–1179.39. Ishigaki T, Kudo SE, Miyachi H, et al. Treatment policy for colonic laterally spreading tumors based on each clinicopathologic feature of 4 subtypes: actual status of pseudo-depressed type. Gastrointest Endosc. 2020; 92:1083–1094.40. Vosko S, Shahidi N, Sidhu M, et al. Optical evaluation for predicting cancer in large nonpedunculated colorectal polyps is accurate for flat lesions. Clin Gastroenterol Hepatol. 2021; 19:2425–2434.41. Smith SCL, Siau K, Cannatelli R, et al. Training methods in optical diagnosis and characterization of colorectal polyps: a systematic review and meta-analysis. Endosc Int Open. 2021; 9:E716–E726.42. Klare P, Haller B, Wormbt S, et al. Narrow-band imaging vs. high definition white light for optical diagnosis of small colorectal polyps: a randomized multicenter trial. Endoscopy. 2016; 48:909–915.43. Takemura Y, Yoshida S, Tanaka S, et al. Computer-aided system for predicting the histology of colorectal tumors by using narrow-band imaging magnifying colonoscopy (with video). Gastrointest Endosc. 2012; 75:179–185.44. Takeda K, Kudo SE, Mori Y, et al. Accuracy of diagnosing invasive colorectal cancer using computer-aided endocytoscopy. Endoscopy. 2017; 49:798–802.45. Ştefănescu D, Streba C, Cârţână ET, et al. Computer aided diagnosis for confocal laser endomicroscopy in advanced colorectal adenocarcinoma. PLoS One. 2016; 11:e0154863.46. Lui TK, Wong KK, Mak LL, et al. Endoscopic prediction of deeply submucosal invasive carcinoma with use of artificial intelligence. Endosc Int Open. 2019; 7:E514–E520.47. Luo X, Wang J, Han Z, et al. Artificial intelligence-enhanced white-light colonoscopy with attention guidance predicts colorectal cancer invasion depth. Gastrointest Endosc. 2021; 94:627–638.48. Tokunaga M, Matsumura T, Nankinzan R, et al. Computer-aided diagnosis system using only white-light endoscopy for the prediction of invasion depth in colorectal cancer. Gastrointest Endosc. 2021; 93:647–653.49. Ito N, Kawahira H, Nakashima H, et al. Endoscopic diagnostic support system for cT1b colorectal cancer using deep learning. Oncology. 2019; 96:44–50.50. Nakajima Y, Zhu X, Nemoto D, et al. Diagnostic performance of artificial intelligence to identify deeply invasive colorectal cancer on non-magnified plain endoscopic images. Endosc Int Open. 2020; 8:E1341–E1348.51. Lu Z, Xu Y, Yao L, et al. Real-time automated diagnosis of colorectal cancer invasion depth using a deep learning model with multimodal data (with video). Gastrointest Endosc. 2022; 95:1186–1194.52. Ang TL, Lim JF, Chua TS, et al. Clinical guidance on endoscopic management of colonic polyps in Singapore. Singapore Med J. 2022; 63:173–186.53. Barel F, Auffret A, Cariou M, et al. High reproducibility is attainable in assessing histoprognostic parameters of pT1 colorectal cancer using routine histopathology slides and immunohistochemistry analyses. Pathology. 2019; 51:46–54.54. Kojima M, Puppa G, Kirsch R, et al. Blood and lymphatic vessel invasion in pT1 colorectal cancer: an international concordance study. J Clin Pathol. 2015; 68:628–632.55. Kouyama Y, Kudo SE, Miyachi H, et al. Practical problems of measuring depth of submucosal invasion in T1 colorectal carcinomas. Int J Colorectal Dis. 2016; 31:137–146.56. Hacking S, Nasim R, Lee L, et al. Whole slide imaging and colorectal carcinoma: a validation study for tumor budding and stromal differentiation. Pathol Res Pract. 2020; 216:153233.57. Hacking S, Angert M, Jin C, et al. Tumor budding in colorectal carcinoma: an institutional interobserver reliability and prognostic study of colorectal adenocarcinoma cases. Ann Diagn Pathol. 2019; 43:151420.58. Martin B, Schäfer E, Jakubowicz E, et al. Interobserver variability in the H&E-based assessment of tumor budding in pT3/4 colon cancer: does it affect the prognostic relevance? Virchows Arch. 2018; 473:189–197.59. Miyachi H, Kudo SE, Ichimasa K, et al. Management of T1 colorectal cancers after endoscopic treatment based on the risk stratification of lymph node metastasis. J Gastroenterol Hepatol. 2016; 31:1126–1132.60. Nakadoi K, Tanaka S, Kanao H, et al. Management of T1 colorectal carcinoma with special reference to criteria for curative endoscopic resection. J Gastroenterol Hepatol. 2012; 27:1057–1062.61. Zwager LW, Bastiaansen BA, Montazeri NS, et al. Deep submucosal invasion is not an independent risk factor for lymph node metastasis in T1 colorectal cancer: a meta-analysis. Gastroenterology. 2022; 163:174–189.62. Goacher E, Randell R, Williams B, et al. The diagnostic concordance of whole slide imaging and light microscopy: a systematic review. Arch Pathol Lab Med. 2017; 141:151–161.63. Koch LH, Lampros JN, Delong LK, et al. Randomized comparison of virtual microscopy and traditional glass microscopy in diagnostic accuracy among dermatology and pathology residents. Hum Pathol. 2009; 40:662–667.64. Mukhopadhyay S, Feldman MD, Abels E, et al. Whole slide imaging versus microscopy for primary diagnosis in surgical pathology: a multicenter blinded randomized noninferiority study of 1992 cases (pivotal study). Am J Surg Pathol. 2018; 42:39–52.65. Weinstein RS, Holcomb MJ, Krupinski EA. Invention and early history of telepathology (1985-2000). J Pathol Inform. 2019; 10:1.66. Ben Hamida A, Devanne M, Weber J, et al. Deep learning for colon cancer histopathological images analysis. Comput Biol Med. 2021; 136:104730.67. Skrede OJ, De Raedt S, Kleppe A, et al. Deep learning for prediction of colorectal cancer outcome: a discovery and validation study. Lancet. 2020; 395:350–360.68. Wang KS, Yu G, Xu C, et al. Accurate diagnosis of colorectal cancer based on histopathology images using artificial intelligence. BMC Med. 2021; 19:76.69. Gupta P, Huang Y, Sahoo PK, et al. Colon tissues classification and localization in whole slide images using deep learning. Diagnostics (Basel). 2021; 11:1398.70. Kwak MS, Lee HH, Yang JM, et al. Deep convolutional neural network-based lymph node metastasis prediction for colon cancer using histopathological images. Front Oncol. 2021; 10:619803.71. Kudo SE, Ichimasa K, Villard B, et al. Artificial intelligence system to determine risk of T1 colorectal cancer metastasis to lymph node. Gastroenterology. 2021; 160:1075–1084.72. Labianca R, Nordlinger B, Beretta GD, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013; 24 Suppl 6:vi64–vi72.73. Kang J, Choi YJ, Kim IK, et al. LASSO-based machine learning algorithm for prediction of lymph node metastasis in T1 colorectal cancer. Cancer Res Treat. 2021; 53:773–783.74. Backes Y, Elias SG, Groen JN, et al. Histologic factors associated with need for surgery in patients with pedunculated T1 colorectal carcinomas. Gastroenterology. 2018; 154:1647–1659.75. Takamatsu M, Yamamoto N, Kawachi H, et al. Prediction of early colorectal cancer metastasis by machine learning using digital slide images. Comput Methods Programs Biomed. 2019; 178:155–161.76. Song JH, Hong Y, Kim ER, et al. Utility of artificial intelligence with deep learning of hematoxylin and eosin-stained whole slide images to predict lymph node metastasis in T1 colorectal cancer using endoscopically resected specimens; prediction of lymph node metastasis in T1 colorectal cancer. J Gastroenterol. 2022; 57:654–666.77. Kasahara K, Katsumata K, Saito A, et al. Artificial intelligence predicts lymph node metastasis or risk of lymph node metastasis in T1 colorectal cancer. Int J Clin Oncol. 2022; 27:1570–1579.78. Brockmoeller S, Echle A, Ghaffari Laleh N, et al. Deep learning identifies inflamed fat as a risk factor for lymph node metastasis in early colorectal cancer. J Pathol. 2022; 256:269–281.79. Echle A, Grabsch HI, Quirke P, et al. Clinical-grade detection of microsatellite instability in colorectal tumors by deep learning. Gastroenterology. 2020; 159:1406–1416.80. Krause J, Grabsch HI, Kloor M, et al. Deep learning detects genetic alterations in cancer histology generated by adversarial networks. J Pathol. 2021; 254:70–79.81. Yamashita R, Long J, Longacre T, et al. Deep learning model for the prediction of microsatellite instability in colorectal cancer: a diagnostic study. Lancet Oncol. 2021; 22:132–141.82. Li JW, Wang LM, Ang TL. Artificial intelligence-assisted colonoscopy: a narrative review of current data and clinical applications. Singapore Med J. 2022; 63:118–124.83. Yang CB, Kim SH, Lim YJ. Preparation of image databases for artificial intelligence algorithm development in gastrointestinal endoscopy. Clin Endosc. 2022; 55:594–604.84. Oliveira SP, Neto PC, Fraga J, et al. CAD systems for colorectal cancer from WSI are still not ready for clinical acceptance. Sci Rep. 2021; 11:14358.85. Peterson E, May FP, Kachikian O, et al. Automated identification and assignment of colonoscopy surveillance recommendations for individuals with colorectal polyps. Gastrointest Endosc. 2021; 94:978–987.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Basics of Deep Learning: A Radiologist's Guide to Understanding Published Radiology Articles on Deep Learning
- Artificial Intelligence in Pathology
- Understanding and Application of Multi-Task Learning in Medical Artificial Intelligence
- Deep Learning in Upper Gastrointestinal Disorders: Status and Future Perspectives
- Deep Learning-Based Artificial Intelligence for Mammography