Ewha Med J.
2023 Dec;46(S1):e33. 10.12771/emj.2023.e33.
Updates on Obesity in Prader-Willi Syndrome: From Genetics to Management
- Affiliations
-
- 1Department of Medical Genetics, Ajou University Hospital, Ajou University School of Medicine, Suwon, Korea
- 2Ewha University-Industry Collaboration Foundation, Seoul, Korea
- KMID: 2550853
- DOI: http://doi.org/10.12771/emj.2023.e33
Abstract
- Prader-Willi syndrome (PWS), which is considered the most common genetic form of obesity, results from the absence of imprinted genes in the paternally derived PWS critical region located on chromosome 15q11.2−13. Infants with PWS exhibit poor sucking, neonatal hypotonia, and delayed motor milestones. These patients begin to experience hyperphagia and obesity from 2 to 3 years of age. PWS is a multisystemic disorder, and its clinical manifestations include developmental delay/ intellectual disability, behavioral problems, dysmorphic facial features, short stature, scoliosis, and endocrine abnormalities such as hypogonadism, growth hormone deficiency, hypothyroidism, and central adrenal insufficiency. Although the underlying mechanism of hyperphagia is not completely understood, hypothalamic and endocrine dysregulation is believed to be responsible for the lack of satiety and abnormal food-seeking behaviors that lead to severe obesity. The management of PWS requires a multidisciplinary team approach. Early diagnosis and comprehensive early intervention are essential to prevent the development of obesity-related morbidities, including metabolic syndrome, diabetes mellitus, obstructive sleep apnea, respiratory failure, pulmonary hypertension, and cardiovascular complications. Although several clinical trials have been conducted on the pharmacologic treatment of obesity in PWS, no drugs have demonstrated a consistently beneficial effect to date. Nevertheless, ongoing research efforts should be directed toward understanding the mechanism of the unique obesity phenotype of PWS and developing pharmacological therapies.
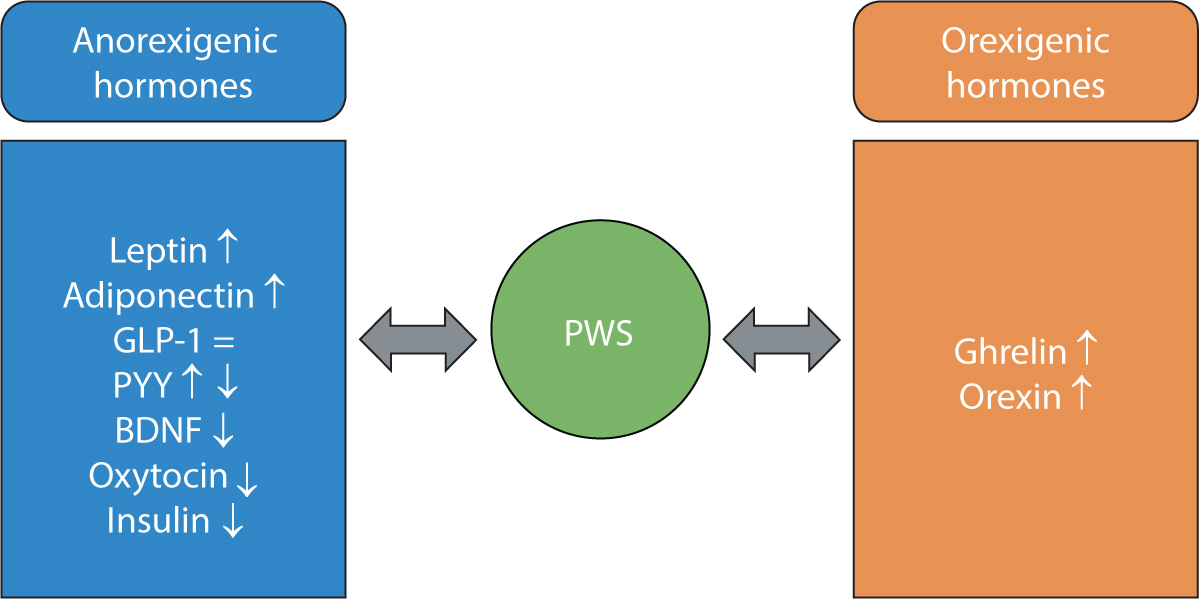
Figure
Reference
-
References
1. Butler MG, Miller JL, Forster JL. Prader-Willi syndrome: clinical genetics, diagnosis and treatment approaches: an update. Curr Pediatr Rev. 2019; 15(4):207–244. DOI: 10.2174/1573396315666190716120925. PMID: 31333129. PMCID: PMC7040524.2. Poitou C, Mosbah H, Clément K. MECHANISMS IN ENDOCRINOLOGY: update on treatments for patients with genetic obesity. Eur J Endocrinol. 2020; 183(5):R149–R166. DOI: 10.1530/EJE-20-0363. PMID: 33107433.3. Butler MG. Prader–Willi syndrome and chromosome 15q11.2 BP1-BP2 region: a review. Int J Mol Sci. 2023; 24(5):4271. DOI: 10.3390/ijms24054271. PMID: 36901699. PMCID: PMC10002205.4. Sohn YB. Genetic obesity: an update with emerging therapeutic approaches. Ann Pediatr Endocrinol Metab. 2022; 27(3):169–175. DOI: 10.6065/apem.2244188.094. PMID: 36203267. PMCID: PMC9537668.5. Angulo MA, Butler MG, Cataletto ME. Prader-Willi syndrome: a review of clinical, genetic, and endocrine findings. J Endocrinol Invest. 2015; 38(12):1249–1263. DOI: 10.1007/s40618-015-0312-9. PMID: 26062517. PMCID: PMC4630255.6. Cassidy SB, Schwartz S, Miller JL, Driscoll DJ. Prader-Willi syndrome. Genet Med. 2012; 14(1):10–26. DOI: 10.1038/gim.0b013e31822bead0. PMID: 22237428.7. Crinò A, Fintini D, Bocchini S, Grugni G. Obesity management in Prader–Willi syndrome: current perspectives. Diabetes Metab Syndr Obes. 2018; 11:579–593. DOI: 10.2147/DMSO.S141352. PMID: 30323638. PMCID: PMC6175547.8. Kim SJ, Cho SY, Jin DK. Prader-Willi syndrome: an update on obesity and endocrine problems. Ann Pediatr Endocrinol Metab. 2021; 26(4):227–236. DOI: 10.6065/apem.2142164.082. PMID: 34991300. PMCID: PMC8749024.9. Manzardo AM, Loker J, Heinemann J, Loker C, Butler MG. Survival trends from the Prader–Willi syndrome association (USA) 40-year mortality survey. Genet Med. 2018; 20(1):24–30. DOI: 10.1038/gim.2017.92. PMID: 28682308. PMCID: PMC5756527.10. Hedgeman E, Ulrichsen SP, Carter S, Kreher NC, Malobisky KP, Braun MM, et al. Long-term health outcomes in patients with Prader–Willi syndrome: a nationwide cohort study in Denmark. Int J Obes. 2017; 41(10):1531–1538. DOI: 10.1038/ijo.2017.139. PMID: 28634363.11. Kim MS, Kim J, Cho J, Cho SY, Jin DK. Tailored management of life-threatening complications related to severe obesity in a young adult with Prader-Willi syndrome. Ann Pediatr Endocrinol Metab. 2022; 27(2):148–152. DOI: 10.6065/apem.2142022.011. PMID: 34670069. PMCID: PMC9260376.12. Kim M, Kim J. Cardiometabolic risk factors and metabolic syndrome based on severity of obesity in Korean children and adolescents: data from the Korea National Health and Nutrition Examination Survey 2007–2018. Ann Pediatr Endocrinol Metab. 2022; 27(4):289–299. DOI: 10.6065/apem.2142230.115. PMID: 35718891. PMCID: PMC9816464.13. Bekx MT, Carrel AL, Shriver TC, Li Z, Allen DB. Decreased energy expenditure is caused by abnormal body composition in infants with Prader-Willi syndrome. J Pediatr. 2003; 143(3):372–376. DOI: 10.1067/S0022-3476(03)00386-X. PMID: 14517523.14. Goldstone AP, Thomas EL, Brynes AE, Bell JD, Frost G, Saeed N, et al. Visceral adipose tissue and metabolic complications of obesity are reduced in Prader-Willi syndrome female adults: evidence for novel influences on body fat distribution. J Clin Endocrinol Metab. 2001; 86(9):4330–4338. DOI: 10.1210/jcem.86.9.7814. PMID: 11549670.15. Butler MG. Single gene and syndromic causes of obesity: illustrative examples. Prog Mol Biol Transl Sci. 2016; 140:1–45. DOI: 10.1016/bs.pmbts.2015.12.003. PMID: 27288824. PMCID: PMC7377403.16. Butler MG, Duis J. Chromosome 15 imprinting disorders: genetic laboratory methodology and approaches. Front Pediatr. 2020; 8:154. DOI: 10.3389/fped.2020.00154. PMID: 32478012. PMCID: PMC7235373.17. Butler MG, Hartin SN, Hossain WA, Manzardo AM, Kimonis V, Dykens E, et al. Molecular genetic classification in Prader-Willi syndrome: a multisite cohort study. J Med Genet. 2019; 56(3):149–153. DOI: 10.1136/jmedgenet-2018-105301. PMID: 29730598. PMCID: PMC7387113.18. Strom SP, Hossain WA, Grigorian M, Li M, Fierro J, Scaringe W, et al. A streamlined approach to Prader-Willi and Angelman syndrome molecular diagnostics. Front Genet. 2021; 12:608889. DOI: 10.3389/fgene.2021.608889. PMID: 34046054. PMCID: PMC8148043.19. Rahman QFA, Jufri NF, Hamid A. Hyperphagia in Prader-Willi syndrome with obesity: from development to pharmacological treatment. Intractable Rare Dis Res. 2023; 12(1):5–12. DOI: 10.5582/irdr.2022.01127. PMID: 36873672. PMCID: PMC9976092.20. Cheon CK. Genetics of Prader-Willi syndrome and Prader-Will-like syndrome. Ann Pediatr Endocrinol Metab. 2016; 21(3):126–135. DOI: 10.6065/apem.2016.21.3.126. PMID: 27777904. PMCID: PMC5073158.21. Pace M, Falappa M, Freschi A, Balzani E, Berteotti C, Lo Martire V, et al. Loss of Snord116 impacts lateral hypothalamus, sleep, and food-related behaviors. JCI Insight. 2020; 5(12):e137495. DOI: 10.1172/jci.insight.137495. PMID: 32365348. PMCID: PMC7406246.22. Buiting K. Prader–Willi syndrome and Angelman syndrome. Am J Med Genet C Semin Med Genet. 2010; 154C(3):365–376. DOI: 10.1002/ajmg.c.30273. PMID: 20803659.23. Swaab DF. Prader—Willi syndrome and the hypothalamus. Acta Paediatr. 1997; 86(S423):50–54. DOI: 10.1111/j.1651-2227.1997.tb18369.x. PMID: 9401539.24. Xu M, Zhang Y, von Deneen KM, Zhu H, Gao JH. Brain structural alterations in obese children with and without Prader-Willi syndrome. Hum Brain Mapp. 2017; 38(8):4228–4238. DOI: 10.1002/hbm.23660. PMID: 28543989. PMCID: PMC6866858.25. Polex-Wolf J, Lam BYH, Larder R, Tadross J, Rimmington D, Bosch F, et al. Hypothalamic loss of Snord116 recapitulates the hyperphagia of Prader-Willi syndrome. J Clin Invest. 2018; 128(3):960–969. DOI: 10.1172/JCI97007. PMID: 29376887. PMCID: PMC5824864.26. Dimitropoulos A, Schultz RT. Food-related neural circuitry in Prader-Willi syndrome: response to high-versus low-calorie foods. J Autism Dev Disord. 2008; 38(9):1642–1653. DOI: 10.1007/s10803-008-0546-x. PMID: 18311513.27. Zhang Y, Wang J, Zhang G, Zhu Q, Cai W, Tian J, et al. The neurobiological drive for overeating implicated in Prader–Willi syndrome. Brain Res. 2015; 1620:72–80. DOI: 10.1016/j.brainres.2015.05.008. PMID: 25998539.28. Goldstone AP, Holland AJ, Butler JV, Whittington JE. Appetite hormones and the transition to hyperphagia in children with Prader-Willi syndrome. Int J Obes. 2012; 36(12):1564–1570. DOI: 10.1038/ijo.2011.274. PMID: 22270375.29. Proto C, Romualdi D, Cento RM, Romano C, Campagna G, Lanzone A. Free and total leptin serum levels and soluble leptin receptors levels in two models of genetic obesity: the Prader-Willi and the Down syndromes. Metabolism. 2007; 56(8):1076–1080. DOI: 10.1016/j.metabol.2007.03.016. PMID: 17618952.30. Bueno M, Esteba-Castillo S, Novell R, Giménez-Palop O, Coronas R, Gabau E, et al. Lack of postprandial peak in brain-derived neurotrophic factor in adults with Prader-Willi syndrome. PLoS One. 2016; 11(9):e0163468. DOI: 10.1371/journal.pone.0163468. PMID: 27685845. PMCID: PMC5042477.31. Höybye C, Barkeling B, Espelund U, Petersson M, Thorén M. Peptides associated with hyperphagia in adults with Prader–Willi syndrome before and during GH treatment. Growth Horm IGF Res. 2003; 13(6):322–327. DOI: 10.1016/S1096-6374(03)00077-7. PMID: 14624765.32. Purtell L, Sze L, Loughnan G, Smith E, Herzog H, Sainsbury A, et al. In adults with Prader–Willi syndrome, elevated ghrelin levels are more consistent with hyperphagia than high PYY and GLP-1 levels. Neuropeptides. 2011; 45(4):301–307. DOI: 10.1016/j.npep.2011.06.001. PMID: 21722955.33. Haqq AM, Muehlbauer MJ, Newgard CB, Grambow S, Freemark M. The metabolic phenotype of Prader-Willi syndrome (PWS) in childhood: heightened insulin sensitivity relative to body mass index. J Clin Endocrinol Metab. 2011; 96(1):E225–E232. DOI: 10.1210/jc.2010-1733. PMID: 20962018. PMCID: PMC3038476.34. Feigerlová E, Diene G, Conte-Auriol F, Molinas C, Gennero I, Salles JP, et al. Hyperghrelinemia precedes obesity in Prader-Willi syndrome. J Clin Endocrinol Metab. 2008; 93(7):2800–2805. DOI: 10.1210/jc.2007-2138. PMID: 18460565.35. Kuppens RJ, Diène G, Bakker NE, Molinas C, Faye S, Nicolino M, et al. Elevated ratio of acylated to unacylated ghrelin in children and young adults with Prader–Willi syndrome. Endocrine. 2015; 50(3):633–642. DOI: 10.1007/s12020-015-0614-x. PMID: 25989955. PMCID: PMC4662713.36. Beauloye V, Diene G, Kuppens R, Zech F, Winandy C, Molinas C, et al. High unacylated ghrelin levels support the concept of anorexia in infants with Prader-Willi syndrome. Orphanet J Rare Dis. 2016; 11(1):56. DOI: 10.1186/s13023-016-0440-0. PMID: 27146407. PMCID: PMC4855494.37. Manzardo AM, Johnson L, Miller JL, Driscoll DJ, Butler MG. Higher plasma orexin a levels in children with Prader–Willi syndrome compared with healthy unrelated sibling controls. Am J Med Genet A. 2016; 170(9):2328–2333. DOI: 10.1002/ajmg.a.37777. PMID: 27518917. PMCID: PMC6697081.38. Butler MG. Management of obesity in Prader–Willi syndrome. Nat Clin Pract Endocrinol Metab. 2006; 2(11):592–593. DOI: 10.1038/ncpendmet0320. PMID: 17082801. PMCID: PMC6791119.39. Miller JL, Linville TD, Dykens EM. Effects of metformin in children and adolescents with Prader-Willi syndrome and early-onset morbid obesity: a pilot study. J Pediatr Endocrinol Metab. 2014; 27((1-2)):23–29. DOI: 10.1515/jpem-2013-0116. PMID: 23893676. PMCID: PMC3864175.40. Hochberg I, Hochberg Z. Expanding the definition of hypothalamic obesity. Obes Rev. 2010; 11(10):709–721. DOI: 10.1111/j.1467-789X.2010.00727.x. PMID: 20233310.41. Kim GW, Lin JE, Blomain ES, Waldman SA. Antiobesity pharmacotherapy: new drugs and emerging targets. Clin Pharmacol Ther. 2014; 95(1):53–66. DOI: 10.1038/clpt.2013.204. PMID: 24105257. PMCID: PMC4054704.42. Fidler MC, Sanchez M, Raether B, Weissman NJ, Smith SR, Shanahan WR, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011; 96(10):3067–3077. DOI: 10.1210/jc.2011-1256. PMID: 21795446.43. Höybye C. Growth hormone treatment of Prader–Willi syndrome has long-term, positive effects on body composition. Acta Paediatr. 2015; 104(4):422–427. DOI: 10.1111/apa.12898. PMID: 25557351.44. Deal CL, Tony M, Höybye C, Allen DB, Tauber M, Christiansen JS. Growth hormone research society workshop summary: consensus guidelines for recombinant human growth hormone therapy in Prader-Willi syndrome. J Clin Endocrinol Metab. 2013; 98(6):E1072–E1087. DOI: 10.1210/jc.2012-3888. PMID: 23543664. PMCID: PMC3789886.45. Carrel AL, Myers SE, Whitman BY, Eickhoff J, Allen DB. Long-term growth hormone therapy changes the natural history of body composition and motor function in children with Prader-Willi syndrome. J Clin Endocrinol Metab. 2010; 95(3):1131–1136. DOI: 10.1210/jc.2009-1389. PMID: 20061431. PMCID: PMC2841537.46. Myers SE, Whitman BY, Carrel AL, Moerchen V, Bekx MT, Allen DB. Two years of growth hormone therapy in young children with Prader–Willi syndrome: physical and neurodevelopmental benefits. Am J Med Genet A. 2007; 143A(5):443–448. DOI: 10.1002/ajmg.a.31468. PMID: 17103437.47. De Waele K, Ishkanian SL, Bogarin R, Miranda CA, Ghatei MA, Bloom SR, et al. Long-acting octreotide treatment causes a sustained decrease in ghrelin concentrations but does not affect weight, behaviour and appetite in subjects with Prader–Willi syndrome. Eur J Endocrinol. 2008; 159(4):381–388. DOI: 10.1530/EJE-08-0462. PMID: 18603572.48. Tan TMM, Vanderpump M, Khoo B, Patterson M, Ghatei MA, Goldstone AP. Somatostatin infusion lowers plasma ghrelin without reducing appetite in adults with Prader-Willi syndrome. J Clin Endocrinol Metab. 2004; 89(8):4162–4165. DOI: 10.1210/jc.2004-0835. PMID: 15292365.49. Consoli A, Çabal Berthoumieu S, Raffin M, Thuilleaux D, Poitou C, Coupaye M, et al. Effect of topiramate on eating behaviours in Prader-Willi syndrome: TOPRADER double-blind randomised placebo-controlled study. Transl Psychiatry. 2019; 9(1):274. DOI: 10.1038/s41398-019-0597-0. PMID: 31685813. PMCID: PMC6828670.50. Kim YM, Lee YJ, Kim SY, Cheon CK, Lim HH. Successful rapid weight reduction and the use of liraglutide for morbid obesity in adolescent Prader-Willi syndrome. Ann Pediatr Endocrinol Metab. 2020; 25(1):52–56. DOI: 10.6065/apem.2020.25.1.52. PMID: 32252218. PMCID: PMC7136503.51. Salehi P, Hsu I, Azen CG, Mittelman SD, Geffner ME, Jeandron D. Effects of exenatide on weight and appetite in overweight adolescents and young adults with Prader-Willi syndrome. Pediatr Obes. 2017; 12(3):221–228. DOI: 10.1111/ijpo.12131. PMID: 27071367. PMCID: PMC5288290.52. Senda M, Ogawa S, Nako K, Okamura M, Sakamoto T, Ito S. The glucagon-like peptide-1 analog liraglutide suppresses ghrelin and controls diabetes in a patient with Prader-Willi syndrome. Endocr J. 2012; 59(10):889–894. DOI: 10.1507/endocrj.EJ12-0074. PMID: 22785236.53. Fintini D, Grugni G, Brufani C, Bocchini S, Cappa M, Crinò A. Use of GLP-1 receptor agonists in Prader-Willi syndrome: report of six cases. Diabetes Care. 2014; 37(4):e76–e77. DOI: 10.2337/dc13-2575. PMID: 24652737.54. Delhanty PJD, Sun Y, Visser JA, Van Kerkwijk A, Huisman M, Van Ijcken WFJ, et al. Unacylated ghrelin rapidly modulates lipogenic and insulin signaling pathway gene expression in metabolically active tissues of GHSR deleted mice. PLoS One. 2010; 5(7):e11749. DOI: 10.1371/journal.pone.0011749. PMID: 20668691. PMCID: PMC2909919.55. Allas S, Caixàs A, Poitou C, Coupaye M, Thuilleaux D, Lorenzini F, et al. AZP-531, an unacylated ghrelin analog, improves food-related behavior in patients with Prader-Willi syndrome: a randomized placebo-controlled trial. PLoS One. 2018; 13(1):e0190849. DOI: 10.1371/journal.pone.0190849. PMID: 29320575. PMCID: PMC5761957.56. Cleverdon ER, McGovern-Gooch KR, Hougland JL. The octanoylated energy regulating hormone ghrelin: an expanded view of ghrelin's biological interactions and avenues for controlling ghrelin signaling. Mol Membr Biol. 2016; 33((6-8)):111–124. DOI: 10.1080/09687688.2017.1388930. PMID: 29143554.57. Hougland JL. Ghrelin octanoylation by ghrelin O-acyltransferase: unique protein biochemistry underlying metabolic signaling. Biochem Soc Trans. 2019; 47(1):169–178. DOI: 10.1042/BST20180436. PMID: 30626708.58. Markham A. Setmelanotide: first approval. Drugs. 2021; 81(3):397–403. DOI: 10.1007/s40265-021-01470-9. PMID: 33638809.59. Hinney A, Körner A, Fischer-Posovszky P. The promise of new anti-obesity therapies arising from knowledge of genetic obesity traits. Nat Rev Endocrinol. 2022; 18(10):623–637. DOI: 10.1038/s41574-022-00716-0. PMID: 35902734. PMCID: PMC9330928.60. Haws R, Brady S, Davis E, Fletty K, Yuan G, Gordon G, et al. Effect of setmelanotide, a melanocortin-4 receptor agonist, on obesity in Bardet-Biedl syndrome. Diabetes Obes Metab. 2020; 22(11):2133–2140. DOI: 10.1111/dom.14133. PMID: 32627316. PMCID: PMC7689750.61. Haws RM, Gordon G, Han JC, Yanovski JA, Yuan G, Stewart MW. The efficacy and safety of setmelanotide in individuals with Bardet-Biedl syndrome or Alström syndrome: phase 3 trial design. Contemp Clin Trials Commun. 2021; 22:100780. DOI: 10.1016/j.conctc.2021.100780. PMID: 34013094. PMCID: PMC8114053.62. Kimonis V, Surampalli A, Wencel M, Gold JA, Cowen NM. A randomized pilot efficacy and safety trial of diazoxide choline controlled-release in patients with Prader-Willi syndrome. PLoS One. 2019; 14(9):e0221615. DOI: 10.1371/journal.pone.0221615. PMID: 31545799. PMCID: PMC6756513.63. Kabasakalian A, Ferretti CJ, Hollander E. Oxytocin and Prader-Willi syndrome. Curr Top Behav Neurosci. 2018; 35:529–557. DOI: 10.1007/7854_2017_28. PMID: 28956320.64. Dykens EM, Miller J, Angulo M, Roof E, Reidy M, Hatoum HT, et al. Intranasal carbetocin reduces hyperphagia in individuals with Prader-Willi syndrome. JCI Insight. 2018; 3(12):e98333. DOI: 10.1172/jci.insight.98333. PMID: 29925684. PMCID: PMC6124421.65. McCandless SE, Yanovski JA, Miller J, Fu C, Bird LM, Salehi P, et al. Effects of MetAP2 inhibition on hyperphagia and body weight in Prader–Willi syndrome: a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2017; 19(12):1751–1761. DOI: 10.1111/dom.13021. PMID: 28556449. PMCID: PMC5673540.66. Burkey BF, Hoglen NC, Inskeep P, Wyman M, Hughes TE, Vath JE. Preclinical efficacy and safety of the novel antidiabetic, antiobesity MetAP2 inhibitor ZGN-1061. J Pharmacol Exp Ther. 2018; 365(2):301–313. DOI: 10.1124/jpet.117.246272. PMID: 29491038.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- One Case of the Prader-Willi Syndrome
- Experience of severe desaturation during anesthetic induction period in an obese adult patient with Prader-Willi syndrome: A case report
- A Case of Prader Willi syndrome
- Anesthetic Management in Pediatric Patient with Prader-Willi Syndrome: A case report
- A Case of Prader-Willi Syndrome