Obstet Gynecol Sci.
2024 Jan;67(1):86-93. 10.5468/ogs.23061.
Comparison of International Ovarian Tumor Analysis ADNEX model and Ovarian-Adnexal Reporting and Data System with final histological diagnosis in adnexal masses: a retrospective study
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Beypazari State Hospital, Ankara, Turkey
- 2Department of Infertility and Reproductive Medicine, Ankara Bilkent City Hospital, Ankara, Turkey
- 3Department of Obstetrics and Gynecology, Ankara Bilkent City Hospital, Ankara, Turkey
- KMID: 2550489
- DOI: http://doi.org/10.5468/ogs.23061
Abstract
Objective
The International ovarian tumor analysis (IOTA)-Assessment of Different NEoplasias in the adneXa (ADNEX) model and the ovarian-adnexal reporting and data system (O-RADS) were developed to improve the diagnostic accuracy of adnexal masses in the preoperative period. This study aimed to evaluate the predictive values of both models in patients who underwent surgery for an adnexal mass at our hospital, based on the final pathological results.
Methods
This study included patients who underwent surgery for adnexal masses at our hospital between 2019 and 2021 and met the inclusion criteria. The IOTA ADNEX model and O-RADS scores were calculated preoperatively.
Results
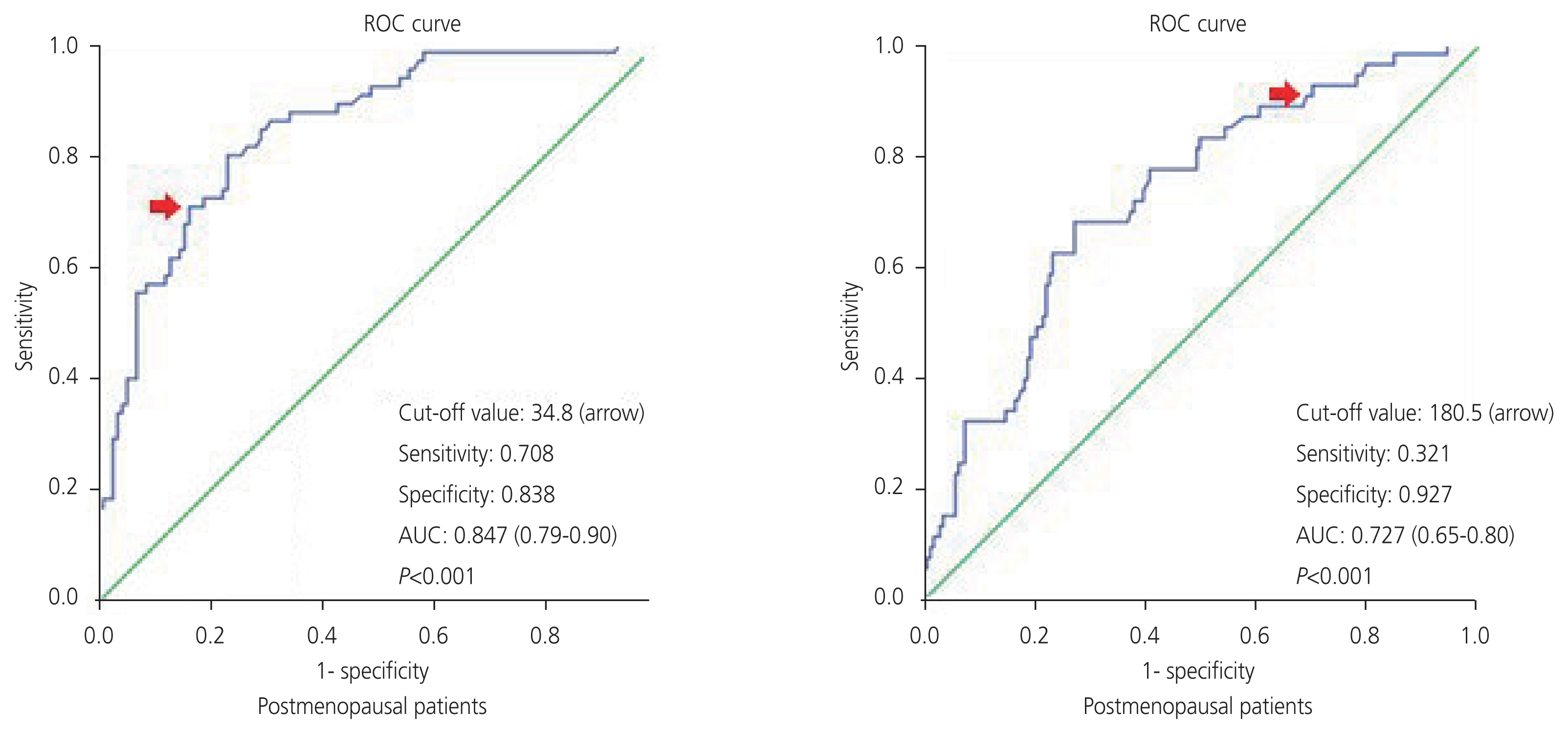
Of the 413 patients, 295 were diagnosed with benign tumors and 118 were diagnosed with malignant tumors. The mean cancer antigen 125 (CA-125) levels for patients diagnosed with benign and malignant were 15.2 unit/mL and 72.5 unit/mL, respectively. According to the receiver operator characteristic analysis for serum CA-125 in postmenopausal and premenopausal patients, the cutoff value of 34.8 unit/mL had a sensitivity of 70.8% and specificity of 83.8% and 180.5 unit/mL had a sensitivity of 32.1% and a specificity of 92.7%, respectively (P<0.001). The sensitivity and specificity values of the IOTA ADNEX model and O-RADS were found as 78.8-48.3% and 97.9-93.5% respectively (P<0.001). There was moderate agreement between the IOTA ADNEX model and O-RADS (Kappa=0.53).
Conclusion
The IOTA ADNEX model has a similar specificity to the O-RADS in malignancy risk assessment, but the sensitivity of the IOTA ADNEX model is higher than that of the O-RADS. The IOTA-ADNEX model can help avoid unnecessary surgeries.
Keyword
Figure
Reference
-
References
1. Yazbek J, Raju SK, Ben-Nagi J, Holland TK, Hillaby K, Jurkovic D. Effect of quality of gynaecological ultrasonography on management of patients with suspected ovarian cancer: a randomised controlled trial. Lancet Oncol. 2008; 9:124–31.2. du Bois A, Rochon J, Pfisterer J, Hoskins WJ. Variations in institutional infrastructure, physician specialization and experience, and outcome in ovarian cancer: a systematic review. Gynecol Oncol. 2009; 112:422–36.3. Chan JK, Kapp DS, Shin JY, Husain A, Teng NN, Berek JS, et al. Influence of the gynecologic oncologist on the survival of ovarian cancer patients. Obstet Gynecol. 2007; 109:1342–50.4. Fung-Kee-Fung M, Kennedy EB, Biagi J, Colgan T, D’Souza D, Elit LM, et al. The optimal organization of gynecologic oncology services: a systematic review. Curr Oncol. 2015; 22:e282–93.5. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.6. Cancer stat facts: ovarian cancer [Internet]. Bethesda: National Cancer Institute;c2022. [cited 2022 Feb 17]. Available from: https://seer.cancer.gov/statfacts/html/ovary.html .7. Bell R, Petticrew M, Sheldon T. The performance of screening tests for ovarian cancer: results of a systematic review. Br J Obstet Gynaecol. 1998; 105:1136–47.8. Andreotti RF, Timmerman D, Strachowski LM, Froyman W, Benacerraf BR, Bennett GL, et al. O-RADS US risk stratification and management system: a consensus guideline from the ACR ovarian-adnexal reporting and data system committee. Radiology. 2020; 294:168–85.9. Van Calster B, Van Hoorde K, Valentin L, Testa AC, Fischerova D, van Holsbeke C, et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ. 2014; 349:g5920.10. Nam G, Lee SR, Jeong K, Kim SH, Moon HS, Chae HD. Assessment of different NEoplasias in the adneXa model for differentiation of benign and malignant adnexal masses in Korean women. Obstet Gynecol Sci. 2021; 64:293–9.11. Bast RC Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981; 68:1331–7.12. Sehouli J, Akdogan Z, Heinze T, Könsgen D, Stengel D, Mustea A, et al. Preoperative determination of CASA (cancer associated serum antigen) and CA-125 for the discrimination between benign and malignant pelvic tumor mass: a prospective study. Anticancer Res. 2003; 23:1115–8.13. Alcázar JL, Errasti T, Zornoza A, Mínguez JA, Galán MJ. Transvaginal color Doppler ultrasonography and CA-125 in suspicious adnexal masses. Int J Gynaecol Obstet. 1999; 66:255–61.14. Antonić J, Rakar S. Validity of colour and pulsed Doppler US and tumour marker CA 125 in differentiation between benign and malignant ovarian masses. Eur J Gynaecol Oncol. 1996; 17:29–35.15. Jacobs IJ, Skates S, Davies AP, Woolas RP, Jeyerajah A, Weidemann P, et al. Risk of diagnosis of ovarian cancer after raised serum CA 125 concentration: a prospective cohort study. BMJ. 1996; 313:1355–8.16. Practice bulletin No. 174: evaluation and management of adnexal masses. Obstet Gynecol. 2016; 128:e210–26.17. Maggino T, Gadducci A, D’Addario V, Pecorelli S, Lissoni A, Stella M, et al. Prospective multicenter study on CA 125 in postmenopausal pelvic masses. Gynecol Oncol. 1994; 54:117–23.18. Xie WT, Wang YQ, Xiang ZS, Du ZS, Huang SX, Chen YJ, et al. Efficacy of IOTA simple rules, O-RADS, and CA125 to distinguish benign and malignant adnexal masses. J Ovarian Res. 2022; 15:15.19. Zhang M, Cheng S, Jin Y, Zhao Y, Wang Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim Biophys Acta Rev Cancer. 2021; 1875:188503.20. Karlsen MA, Sandhu N, Høgdall C, Christensen IJ, Nedergaard L, Lundvall L, et al. Evaluation of HE4, CA125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) as diagnostic tools of epithelial ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2012; 127:379–83.21. Kang KN, Koh EY, Jang JY, Kim CW. Multiple biomarkers are more accurate than a combination of carbohydrate antigen 125 and human epididymis protein 4 for ovarian cancer screening. Obstet Gynecol Sci. 2022; 65:346–54.22. Van Calster B, Van Hoorde K, Froyman W, Kaijser J, Wynants L, Landolfo C, et al. Practical guidance for applying the ADNEX model from the IOTA group to discriminate between different subtypes of adnexal tumors. Facts Views Vis Obgyn. 2015; 7:32–41.23. Jeong SY, Park BK, Lee YY, Kim TJ. Validation of iotaadnex model in discriminating characteristics of adnexal masses: a comparison with subjective assessment. J Clin Med. 2020; 9:2010.24. Cao L, Wei M, Liu Y, Fu J, Zhang H, Huang J, et al. Validation of American College of Radiology ovarian-adnexal reporting and data system ultrasound (O-RADS US): analysis on 1054 adnexal masses. Gynecol Oncol. 2021; 162:107–12.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Assessment of different NEoplasias in the adneXa model for differentiation of benign and malignant adnexal masses in Korean women
- Adnexal Masses: Clinical Application of Multiparametric MR Imaging & O-RADS MRI
- Application of O-RADS US combined with MV-Flow to diagnose ovarian-adnexal tumors
- Comparison of Doppler Waveform Index in Benign and Malignant Ovarian Tumor
- Diagnostic Efficacy of the Morphological Scoring System of Ultrasound, CA-125, Color Doppler Sonography(CDS), and Magnetic Resonance Imaging(MRI) in Detecting Ovarian Malignancy