J Korean Neurosurg Soc.
2024 Jan;67(1):103-114. 10.3340/jkns.2023.0143.
Risk Factor Analysis of Cryopreserved Autologous Bone Flap Resorption in Adult Patients Undergoing Cranioplasty with Volumetry Measurement Using Conventional Statistics and Machine-Learning Technique
- Affiliations
-
- 1Department of Neurosurgery, Dankook University Hospital, Cheonan, Korea
- 2Department of Neurosurgery, College of Medicine, Dankook University, Cheonan, Korea
- KMID: 2550464
- DOI: http://doi.org/10.3340/jkns.2023.0143
Abstract
Objective
: Decompressive craniectomy (DC) with duroplasty is one of the common surgical treatments for life-threatening increased intracranial pressure (ICP). Once ICP is controlled, cranioplasty (CP) with reinsertion of the cryopreserved autologous bone flap or a synthetic implant is considered for protection and esthetics. Although with the risk of autologous bone flap resorption (BFR), cryopreserved autologous bone flap for CP is one of the important material due to its cost effectiveness. In this article, we performed conventional statistical analysis and the machine learning technique understand the risk factors for BFR.
Methods
: Patients aged >18 years who underwent autologous bone CP between January 2015 and December 2021 were reviewed. Demographic data, medical records, and volumetric measurements of the autologous bone flap volume from 94 patients were collected. BFR was defined with absolute quantitative method (BFR-A) and relative quantitative method (BFR%). Conventional statistical analysis and random forest with hyper-ensemble approach (RF with HEA) was performed. And overlapped partial dependence plots (PDP) were generated.
Results
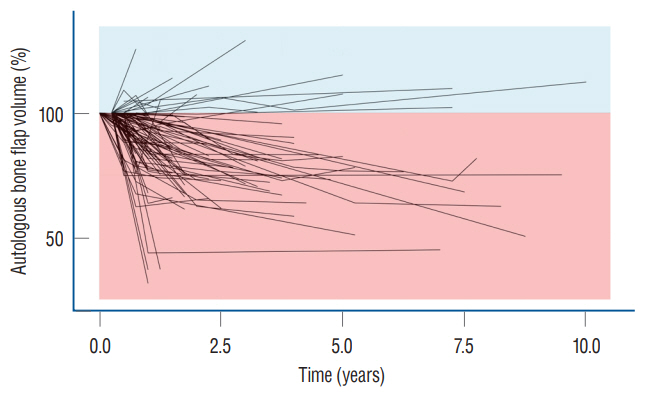
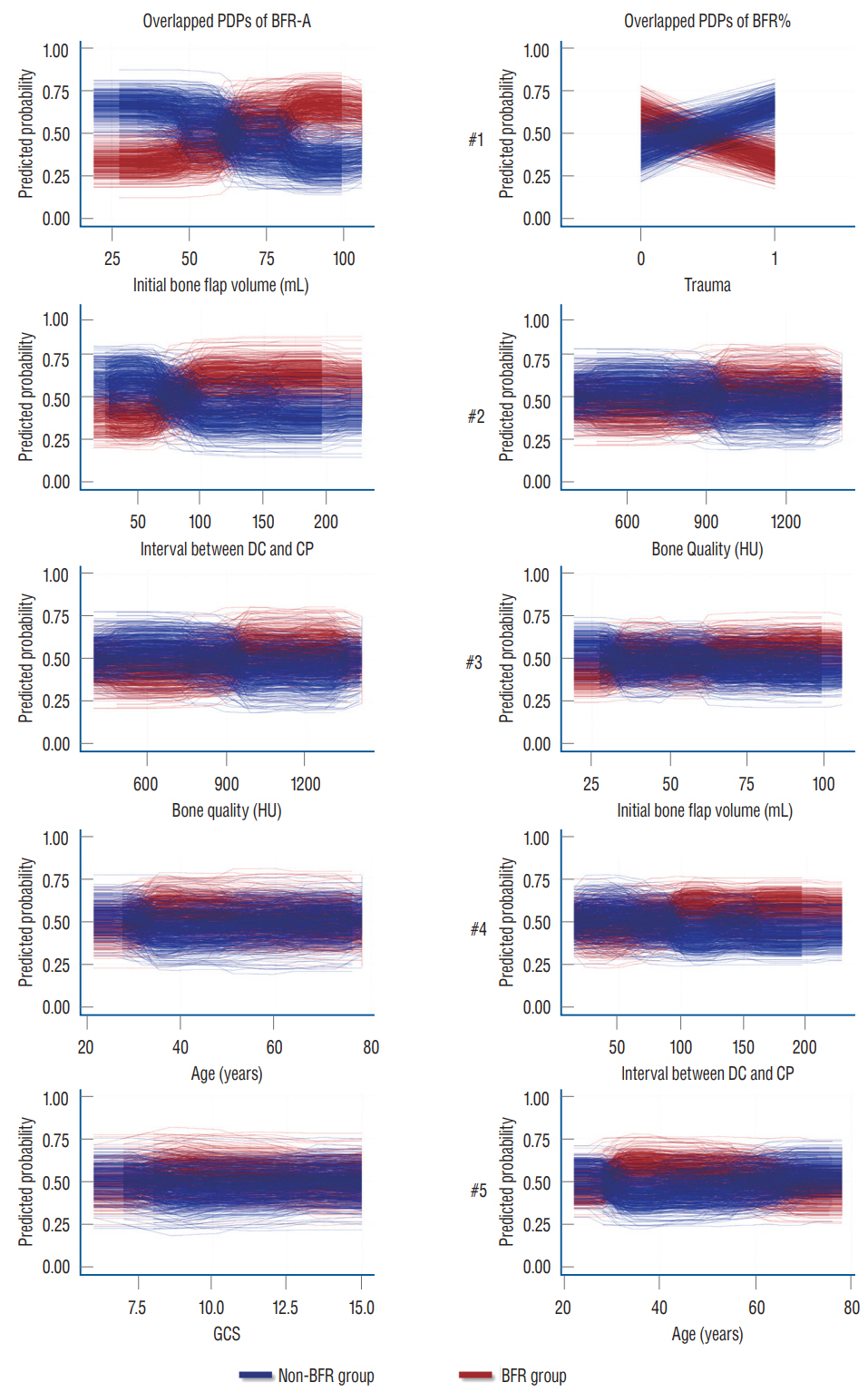
: Conventional statistical analysis showed that only the initial autologous bone flap volume was statistically significant on BFR-A. RF with HEA showed that the initial autologous bone flap volume, interval between DC and CP, and bone quality were the factors with most contribution to BFR-A, while, trauma, bone quality, and initial autologous bone flap volume were the factors with most contribution to BFR%. Overlapped PDPs of the initial autologous bone flap volume on the BRF-A crossed at approximately 60 mL, and a relatively clear separation was found between the non-BFR and BFR groups. Therefore, the initial autologous bone flap of over 60 mL could be a possible risk factor for BFR.
Conclusion
: From the present study, BFR in patients who underwent CP with autologous bone flap might be inevitable. However, the degree of BFR may differ from one to another. Therefore, considering artificial bone flaps as implants for patients with large DC could be reasonable. Still, the risk factors for BFR are not clearly understood. Therefore, chronological analysis and pathophysiologic studies are needed.
Figure
Cited by 1 articles
-
Editors’ Pick in January 2024
Hee-Jin Yang
J Korean Neurosurg Soc. 2024;67(1):1-2. doi: 10.3340/jkns.2023.0254.
Reference
-
References
1. Aguilaniu B, Hess D, Kelkel E, Briault A, Destors M, Boutros J, et al. A machine learning approach to predict extreme inactivity in COPD patients using non-activity-related clinical data. PLoS One. 16:e0255977. 2021.
Article2. Anderson J. Decompressive craniectomy in diffuse traumatic brain Injury : Cooper DJ, Rosenfeld JV, Murray L, et al. N Engl J Med 2011; 364: 1493-502. J Emerg Med. 41:450. 2011.3. Ashayeri K, Jackson EM, Huang J, Brem H, Gordon CR. Syndrome of the trephined: a systematic review. Neurosurgery. 79:525–534. 2016.4. Barzaghi LR, Parisi V, Gigliotti CR, Giudice L, Snider S, Dell’Acqua A, et al. Bone resorption in autologous cryopreserved cranioplasty: quantitative evaluation, semiquantitative score and clinical significance. Acta Neurochir (Wien). 161:483–491. 2019.
Article5. Beez T, Munoz-Bendix C, Ahmadi SA, Steiger HJ, Beseoglu K. From decompressive craniectomy to cranioplasty and beyond-a pediatric neurosurgery perspective. Childs Nerv Syst. 35:1517–1524. 2019.
Article6. Bhaskar IP, Yusheng L, Zheng M, Lee GY. Autogenous skull flaps stored frozen for more than 6 months: do they remain viable? J Clin Neurosci. 18:1690–1693. 2011.
Article7. Bowers CA, Riva-Cambrin J, Hertzler DA 2nd, Walker ML. Risk factors and rates of bone flap resorption in pediatric patients after decompressive craniectomy for traumatic brain injury. J Neurosurg Pediatr. 11:526–532. 2013.
Article8. Breiman L. Random forests. Mach Learn. 45:5–32. 2001.9. Brommeland T, Rydning PN, Pripp AH, Helseth E. Cranioplasty complications and risk factors associated with bone flap resorption. Scand J Trauma Resusc Emerg Med. 23:75. 2015.
Article10. Broughton E, Pobereskin L, Whitfield PC. Seven years of cranioplasty in a regional neurosurgical centre. Br J Neurosurg. 28:34–39. 2014.
Article11. Chung J, Cheong JH, Kim JM, Lee DH, Yi HJ, Choi KS, et al. Is fetal-type posterior cerebral artery a risk factor for recurrence in coiled internal carotid artery-incorporating posterior communicating artery aneurysms? Analysis of conventional statistics, computational fluid dynamics, and random forest with hyper-ensemble approach. Neurosurgery. 93:611–621. 2023.
Article12. Coulter IC, Pesic-Smith JD, Cato-Addison WB, Khan SA, Thompson D, Jenkins AJ, et al. Routine but risky: a multi-centre analysis of the outcomes of cranioplasty in the Northeast of England. Acta Neurochir (Wien). 156:1361–1368. 2014.
Article13. da Costa Benalia VH, Pedrozo CAG, Kormanski MK, Veiga JCE, de Aguiar GB. Spontaneous bone flap resorption following cranioplasty using autologous bone. J Craniofac Surg. 32:293–296. 2021.
Article14. Dabadi S, Dhungel RR, Sharma U, Shrestha D, Gurung P, Shrestha R, et al. Customized cost-effective polymethyl-methacrylate cranioplasty implant using three-dimensional printer. Asian J Neurosurg. 16:150–154. 2021.
Article15. Dowlati E, Pasko KBD, Molina EA, Felbaum DR, Mason RB, Mai JC, et al. Decompressive hemicraniectomy and cranioplasty using subcutaneously preserved autologous bone flaps versus synthetic implants: perioperative outcomes and cost analysis. J Neurosurg. 137:1831–1838. 2022.
Article16. Ernst G, Qeadan F, Carlson AP. Subcutaneous bone flap storage after emergency craniectomy: cost-effectiveness and rate of resorption. J Neurosurg. 129:1604–1610. 2018.
Article17. Frassanito P, Massimi L, Caldarelli M, Tamburrini G, Di Rocco C. Complications of delayed cranial repair after decompressive craniectomy in children less than 1 year old. Acta Neurochir (Wien). 154:927–933. 2012.
Article18. Friedman JH. Greedy function approximation: a gradient boosting machine. Ann Stat. 29:1189–1232. 2001.
Article19. Gooch MR, Gin GE, Kenning TJ, German JW. Complications of cranioplasty following decompressive craniectomy: analysis of 62 cases. Neurosurg Focus. 26:E9. 2009.
Article20. Gosain AK, Gosain SA, Sweeney WM, Song LS, Amarante MTJ. Regulation of osteogenesis and survival within bone grafts to the calvaria: the effect of the dura versus the pericranium. Plast Reconstr Surg. 128:85–94. 2011.
Article21. Göttsche J, Mende KC, Schram A, Westphal M, Amling M, Regelsberger J, et al. Cranial bone flap resorption-pathological features and their implications for clinical treatment. Neurosurg Rev. 44:2253–2260. 2021.
Article22. Grant GA, Jolley M, Ellenbogen RG, Roberts TS, Gruss JR, Loeser JD. Failure of autologous bone-assisted cranioplasty following decompressive craniectomy in children and adolescents. J Neurosurg. 100(2 Suppl Pediatrics):163–168. 2004.
Article23. Gupta D. Novel solutions to cranioplasty: from exchange cranioplasty to synthetic patient-specific implants. Neurol India. 69:618–619. 2021.
Article24. Halani SH, Chu JK, Malcolm JG, Rindler RS, Allen JW, Grossberg JA, et al. Effects of cranioplasty on cerebral blood flow following decompressive craniectomy: a systematic review of the literature. Neurosurgery. 81:204–216. 2017.
Article25. Hersh DS, Anderson HJ, Woodworth GF, Martin JE, Khan YM. Bone flap resorption in pediatric patients following autologous cranioplasty. Oper Neurosurg (Hagerstown). 20:436–443. 2021.
Article26. Iwama T, Yamada J, Imai S, Shinoda J, Funakoshi T, Sakai N. The use of frozen autogenous bone flaps in delayed cranioplasty revisited. Neurosurgery. 52:591–596. discussion 595-596. 2003.
Article27. Kim JH, Kim JH, Kwon TH, Chong K, Hwang SY, Yoon WK. Aseptic bone flap resorption after cranioplasty with autologous bone: incidence, risk factors, and clinical implications. World Neurosurg. 115:e111–e118. 2018.
Article28. Kim JK, Lee SB, Yang SY. Cranioplasty using autologous bone versus porous polyethylene versus custom-made titanium mesh : a retrospective review of 108 patients. J Korean Neurosurg Soc. 61:737–746. 2018.
Article29. Korhonen TK, Salokorpi N, Niinimäki J, Serlo W, Lehenkari P, Tetri S. Quantitative and qualitative analysis of bone flap resorption in patients undergoing cranioplasty after decompressive craniectomy. J Neurosurg. 130:312–321. 2018.
Article30. Korhonen TK, Tetri S, Huttunen J, Lindgren A, Piitulainen JM, Serlo W, et al. Predictors of primary autograft cranioplasty survival and resorption after craniectomy. J Neurosurg. 130:1672–1679. 2018.
Article31. Li J, von Campe G, Pepe A, Gsaxner C, Wang E, Chen X, et al. Automatic skull defect restoration and cranial implant generation for cranioplasty. Med Image Anal. 73:102171. 2021.
Article32. Liaw A, Wiener M. Classification and regression by randomForest. R News. 2:18–22. 2002.33. Lunardon N, Menardi G, Torelli N. ROSE: a package for binary imbalanced learning. R J. 6:79–89. 2014.
Article34. Malcolm JG, Rindler RS, Chu JK, Chokshi F, Grossberg JA, Pradilla G, et al. Early cranioplasty is associated with greater neurological improvement: a systematic review and meta-analysis. Neurosurgery. 82:278–288. 2018.
Article35. Martin KD, Franz B, Kirsch M, Polanski W, von der Hagen M, Schackert G, et al. Autologous bone flap cranioplasty following decompressive craniectomy is combined with a high complication rate in pediatric traumatic brain injury patients. Acta Neurochir (Wien). 156:813–824. 2014.
Article36. Na MK, Won YD, Kim CH, Kim JM, Cheong JH, Ryu JI, et al. Opportunistic osteoporosis screening via the measurement of frontal skull Hounsfield units derived from brain computed tomography images. PLoS One. 13:e0197336. 2018.
Article37. Parichay PJ, Khanapure K, Joshi KC, Aniruddha TJ, Sandhya M, Hegde AS. Clinical and radiological assessment of cerebral hemodynamics after cranioplasty for decompressive craniectomy - a clinical study. J Clin Neurosci. 42:97–101. 2017.
Article38. Park SP, Kim JH, Kang HI, Kim DR, Moon BG, Kim JS. Bone flap resorption following cranioplasty with autologous bone: quantitative measurement of bone flap resorption and predictive factors. J Korean Neurosurg Soc. 60:749–754. 2017.
Article39. Petrini A, Mesiti M, Schubach M, Frasca M, Danis D, Re M, et al. parSMURF, a high-performance computing tool for the genome-wide detection of pathogenic variants. GigaScience. 9:giaa052. 2020.
Article40. Piedra MP, Thompson EM, Selden NR, Ragel BT, Guillaume DJ. Optimal timing of autologous cranioplasty after decompressive craniectomy in children. J Neurosurg Pediatr. 10:268–272. 2012.
Article41. Piitulainen JM, Kauko T, Aitasalo KM, Vuorinen V, Vallittu PK, Posti JP. Outcomes of cranioplasty with synthetic materials and autologous bone grafts. World Neurosurg. 83:708–714. 2015.
Article42. Polin RS, Shaffrey ME, Bogaev CA, Tisdale N, Germanson T, Bocchicchio B, et al. Decompressive bifrontal craniectomy in the treatment of severe refractory posttraumatic cerebral edema. Neurosurgery. 41:84–94. discussion 92-94. 1997.
Article43. Rashidi A, Sandalcioglu IE, Luchtmann M. Aseptic bone-flap resorption after cranioplasty - incidence and risk factors. PLoS One. 15:e0228009. 2020.
Article44. Rocque BG, Agee BS, Thompson EM, Piedra M, Baird LC, Selden NR, et al. Complications following pediatric cranioplasty after decompressive craniectomy: a multicenter retrospective study. J Neurosurg Pediatr. 22:225–232. 2018.
Article45. Schoekler B, Trummer M. Prediction parameters of bone flap resorption following cranioplasty with autologous bone. Clin Neurol Neurosurg. 120:64–67. 2014.
Article46. Schubach M, Re M, Robinson PN, Valentini G. Imbalance-aware machine learning for predicting rare and common disease-associated noncoding variants. Sci Rep. 7:2959. 2017.
Article47. Schwab S, Steiner T, Aschoff A, Schwarz S, Steiner HH, Jansen O, et al. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke. 29:1888–1893. 1998.
Article48. Scotter J, Iorga R, Stefanou D, Wilson MH. Management of malignant middle cerebral artery infarction following a cardiac stab wound--the role of early decompressive craniectomy. Br J Neurosurg. 28:534–535. 2014.
Article49. Shah AM, Jung H, Skirboll S. Materials used in cranioplasty: a history and analysis. Neurosurg Focus. 36:E19. 2014.
Article50. Shahid AH, Mohanty M, Singla N, Mittal BR, Gupta SK. The effect of cranioplasty following decompressive craniectomy on cerebral blood perfusion, neurological, and cognitive outcome. J Neurosurg. 128:229–235. 2018.
Article51. Signorelli F, Giordano M, Caccavella VM, Ioannoni E, Gelormini C, Caricato A, et al. A systematic review and meta-analysis of factors involved in bone flap resorption after decompressive craniectomy. Neurosurg Rev. 45:1915–1922. 2022.
Article52. Smedley D, Schubach M, Jacobsen JOB, Köhler S, Zemojtel T, Spielmann M, et al. A whole-genome analysis framework for effective identification of pathogenic regulatory variants in Mendelian disease. Am J Hum Genet. 99:595–606. 2016.
Article53. Sorek S, Miller A, Griepp D, Moawad S, Zanzerkia R, Rahme R. Skull reconstruction using a custom-made, three-dimensional-printed, hydroxyapatite-titanium cranioplasty implant: largest single-center U.S. experience. World Neurosurg. 167:e1387–e1394. 2022.
Article54. Stevenson S, Li XQ, Davy DT, Klein L, Goldberg VM. Critical biological determinants of incorporation of non-vascularized cortical bone grafts. Quantification of a complex process and structure. J Bone Joint Surg Am. 79:1–16. 1997.
Article55. Sultan SM, Davidson EH, Butala P, Schachar JS, Witek L, Szpalski C, et al. Interval cranioplasty: comparison of current standards. Plast Reconstr Surg. 127:1855–1864. 2011.
Article56. Team R Core. R: A language and environment for statistical computing. Available at : https://www.r-project.org/.57. Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard JP, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL trial). Stroke. 38:2506–2517. 2007.
Article58. Voss HU, Heier LA, Schiff ND. Multimodal imaging of recovery of functional networks associated with reversal of paradoxical herniation after cranioplasty. Clin Imaging. 35:253–258. 2011.
Article59. Yoon HG, Ko Y, Kim YS, Bak KH, Chun HJ, Na MK, et al. Efficacy of 3D-printed titanium mesh-type patient-specific implant for cranioplasty. Korean J Neurotrauma. 17:91–99. 2021.
Article60. Zanotti B, Zingaretti N, Verlicchi A, Robiony M, Alfieri A, Parodi PC. Cranioplasty: review of materials. J Craniofac Surg. 27:2061–2072. 2016.61. Zhu S, Chen Y, Lin F, Chen Z, Jiang X, Zhang J, et al. Complications following titanium cranioplasty compared with nontitanium implants cranioplasty: a systematic review and meta-analysis. J Clin Neurosci. 84:66–74. 2021.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Cranioplasty Using Frozen Autologous Bone Following Post-Traumatic Decompressive Craniectomy
- Bone Flap Resorption Following Cranioplasty with Autologous Bone: Quantitative Measurement of Bone Flap Resorption and Predictive Factors
- Resorption of Autogenous Bone Graft in Cranioplasty: Resorption and Reintegration Failure
- Bone Flap Resorption Following Cranioplasty after Decompressive Craniectomy: Preliminary Report
- Bone Resorption of Autologous Cranioplasty Following Decompressive Craniectomy in Children: Case Report