Ann Lab Med.
2024 Jan;44(1):56-63. 10.3343/alm.2024.44.1.56.
Clinical Usefulness of a Cell-based Assay for Detecting Myelin Oligodendrocyte Glycoprotein Antibodies in Central Nervous System Inflammatory Disorders
- Affiliations
-
- 1Department of Neurology, Soonchunhyang University Hospital Cheonan, Soonchunhyang University College of Medicine, Cheonan, Korea
- 2Nuffield Department of Clinical Neurosciences, John Radcliffe Hospital, University of Oxford, Oxford, United Kingdom
- 3Department of Neurology, Neuroscience Center, Samsung Medical Center, Seoul, Korea
- 4Department of Neurology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 5Department of Neurology, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- 6Department of Neurology, Kyungpook National University Chilgok Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
- 7Department of Neurology, Jeju National University School of Medicine, Jeju, Korea
- 8Department of Neurology, Uijeongbu Eulji Medical Center, Eulji University School of Medicine, Uijeongbu, Korea
- 9Department of Neurology, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea
- 10Department of Ophthalmology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 11Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 12Department of Health Sciences and Technology, Samsung Advanced Institute for Health Sciences & Technology (SAIHST), Sungkyunkwan University, Seoul, Korea
- KMID: 2550223
- DOI: http://doi.org/10.3343/alm.2024.44.1.56
Abstract
- Background
The clinical implications of myelin oligodendrocyte glycoprotein autoantibodies (MOG-Abs) are increasing. Establishing MOG-Ab assays is essential for effectively treating patients with MOG-Abs. We established an in-house cell-based assay (CBA) to detect MOG-Abs to identify correlations with patients’ clinical characteristics.
Methods
We established the CBA using HEK 293 cells transiently overexpressing fulllength human MOG, tested it against 166 samples from a multicenter registry of central nervous system (CNS) inflammatory disorders, and compared the results with those of the Oxford MOG-Ab-based CBA and a commercial MOG-Ab CBA kit. We recruited additional patients with MOG-Abs and compared the clinical characteristics of MOG-Ab-associated disease (MOGAD) with those of neuromyelitis optica spectrum disorder (NMOSD).
Results
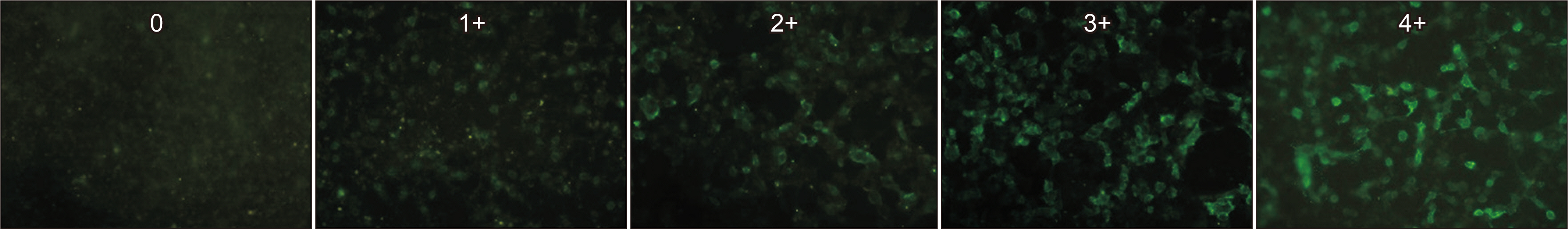
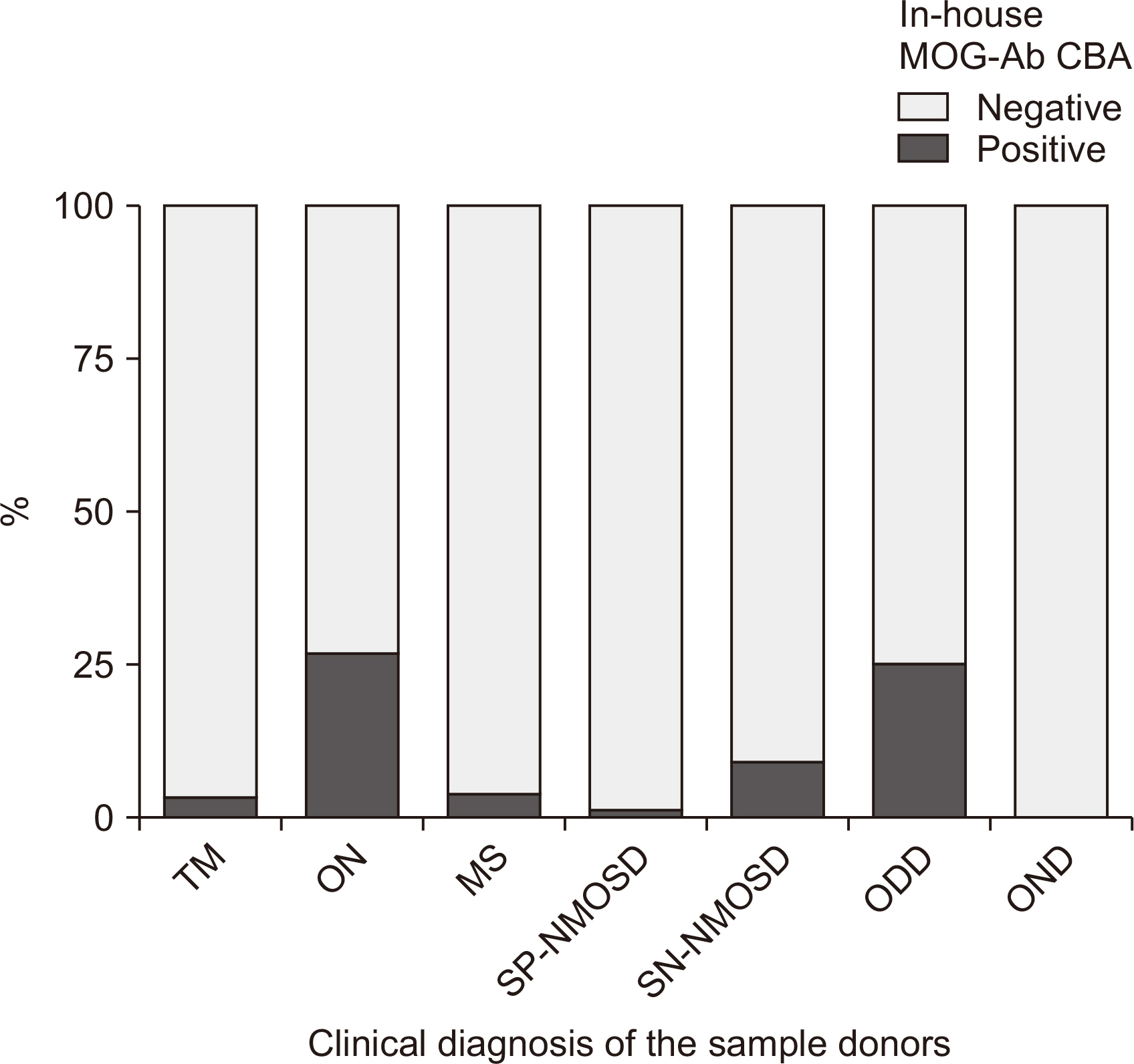
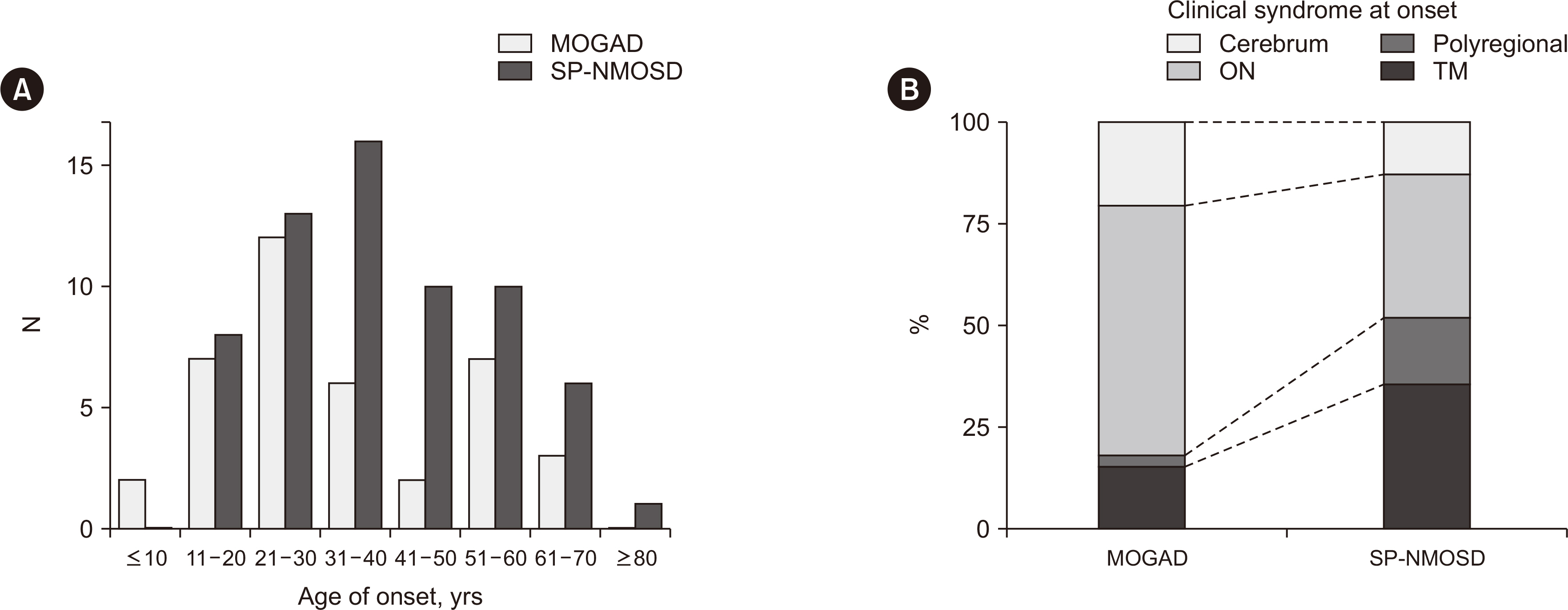
Of 166 samples tested, 10 tested positive for MOG-Abs, with optic neuritis (ON) being the most common manifestation (4/15, 26.7%). The in-house and Oxford MOG-Ab CBAs agreed for 164/166 (98.8%) samples (κ = 0.883, P < 0.001); two patients (2/166, 1.2%) were only positive in our in-house CBA, and the CBA scores of the two laboratories correlated well (r = 0.663, P < 0.001). The commercial MOG-Ab CBA kit showed one falsenegative and three false-positive results. The clinical presentation at disease onset differed between MOGAD and NMOSD; ON was the most frequent manifestation in MOGAD, and transverse myelitis was most frequent in NMOSD.
Conclusions
The in-house CBA for MOG-Abs demonstrated reliable results and can potentially be used to evaluate CNS inflammatory disorders. A comprehensive, long-term study with a large patient population would clarify the clinical significance of MOG-Abs.
Keyword
Figure
Reference
-
1. Prüss H. 2021; Autoantibodies in neurological disease. Nat Rev Immunol. 21:798–813. DOI: 10.1038/s41577-021-00543-w. PMID: 33976421. PMCID: PMC8111372.
Article2. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. 2015; International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 85:177–89. DOI: 10.1212/WNL.0000000000001729. PMID: 26092914. PMCID: PMC4515040.
Article3. Jeyalatha MV, Therese KL, Anand AR. 2022; An update on the laboratory diagnosis of neuromyelitis optica spectrum disorders. J Clin Neurol. 18:152–62. DOI: 10.3988/jcn.2022.18.2.152. PMID: 35274835. PMCID: PMC8926771.
Article4. Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, et al. 2018; MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation. 15:134. DOI: 10.1186/s12974-018-1144-2. PMID: 29724224. PMCID: PMC5932838.
Article5. de Mol CL, Wong Y, van Pelt ED, Wokke B, Siepman T, Neuteboom RF, et al. 2020; The clinical spectrum and incidence of anti-MOG-associated acquired demyelinating syndromes in children and adults. Mult Scler. 26:806–14. DOI: 10.1177/1352458519845112. PMID: 31094288. PMCID: PMC7294530.
Article6. Kim KH, Kim SH, Hyun JW, Kim HJ. 2022; Clinical and radiological features of myelin oligodendrocyte glycoprotein-associated myelitis in adults. J Clin Neurol. 18:280–9. DOI: 10.3988/jcn.2022.18.3.280. PMID: 35589317. PMCID: PMC9163942.
Article7. Akaishi T, Takahashi T, Misu T, Kaneko K, Takai Y, Nishiyama S, et al. 2021; Difference in the source of anti-AQP4-IgG and anti-MOG-IgG antibodies in CSF in patients with neuromyelitis optica spectrum disorder. Neurology. 97:e1–12. DOI: 10.1212/WNL.0000000000012175. PMID: 33980704. PMCID: PMC8312856.
Article8. Sechi E, Krecke KN, Pittock SJ, Dubey D, Lopez-Chiriboga AS, Kunchok A, et al. 2021; Frequency and characteristics of MRI-negative myelitis associated with MOG autoantibodies. Mult Scler. 27:303–8. DOI: 10.1177/1352458520907900. PMID: 32103708. PMCID: PMC7500857.
Article9. Cobo-Calvo A, Ruiz A, Rollot F, Arrambide G, Deschamps R, Maillart E, et al. 2021; Clinical features and risk of relapse in children and adults with myelin oligodendrocyte glycoprotein antibody-associated disease. Ann Neurol. 89:30–41. DOI: 10.1002/ana.25909. PMID: 32959427.
Article10. Hyun JW, Lee HL, Jeong WK, Lee HJ, Shin JH, Min JH, et al. 2021; Comparison of MOG and AQP4 antibody seroprevalence in Korean adults with inflammatory demyelinating CNS diseases. Mult Scler. 27:964–7. DOI: 10.1177/1352458520948213. PMID: 32779521.
Article11. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. 2011; Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 69:292–302. DOI: 10.1002/ana.22366. PMID: 21387374. PMCID: PMC3084507.
Article12. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. 2018; Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 17:162–73. DOI: 10.1016/S1474-4422(17)30470-2. PMID: 29275977.
Article13. Waters P, Woodhall M, O'Connor KC, Reindl M, Lang B, Sato DK, et al. 2015; MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm. 2:e89. DOI: 10.1212/NXI.0000000000000089. PMID: 25821844. PMCID: PMC4370386.14. Waters PJ, Komorowski L, Woodhall M, Lederer S, Majed M, Fryer J, et al. 2019; A multicenter comparison of MOG-IgG cell-based assays. Neurology. 92:e1250–5. DOI: 10.1212/WNL.0000000000007096. PMID: 30728305. PMCID: PMC6511109.
Article15. Kang ES, Min JH, Lee KH, Kim BJ. 2012; Clinical usefulness of cell-based indirect immunofluorescence assay for the detection of aquaporin-4 antibodies in neuromyelitis optica spectrum disorder. Ann Lab Med. 32:331–8. DOI: 10.3343/alm.2012.32.5.331. PMID: 22950068. PMCID: PMC3427820.
Article16. Fujihara K, Cook LJ. 2020; Neuromyelitis optica spectrum disorders and myelin oligodendrocyte glycoprotein antibody-associated disease: current topics. Curr Opin Neurol. 33:300–8. DOI: 10.1097/WCO.0000000000000828. PMID: 32374571.
Article17. Lee HJ, Kim B, Waters P, Woodhall M, Irani S, Ahn S, et al. 2018; Chronic relapsing inflammatory optic neuropathy (CRION): a manifestation of myelin oligodendrocyte glycoprotein antibodies. J Neuroinflammation. 15:302. DOI: 10.1186/s12974-018-1335-x. PMID: 30382857. PMCID: PMC6208174.
Article18. Hamid SHM, Whittam D, Mutch K, Linaker S, Solomon T, Das K, et al. 2017; What proportion of AQP4-IgG-negative NMO spectrum disorder patients are MOG-IgG positive? A cross sectional study of 132 patients. J Neurol. 264:2088–94. DOI: 10.1007/s00415-017-8596-7. PMID: 28840314. PMCID: PMC5617862.
Article19. Reindl M, Di Pauli F, Rostásy K, Berger T. 2013; The spectrum of MOG autoantibody-associated demyelinating diseases. Nat Rev Neurol. 9:455–61. DOI: 10.1038/nrneurol.2013.118. PMID: 23797245.
Article20. Cao Y, Xu J, Yi Z, Zhou L. 2023; A case of MOGAD complicated with cerebral vasculitis: case report and literature review. J Clin Neurol. 19:96–8. DOI: 10.3988/jcn.2023.19.1.96. PMID: 36606653. PMCID: PMC9833881.
Article21. Jurynczyk M, Messina S, Woodhall MR, Raza N, Everett R, Roca-Fernandez A, et al. 2017; Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 140:3128–38. DOI: 10.1093/brain/awx276. PMID: 29136091.
Article22. Kunchok A, Chen JJ, McKeon A, Mills JR, Flanagan EP, Pittock SJ. 2020; Coexistence of myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies in adult and pediatric patients. JAMA Neurol. 77:257–9. DOI: 10.1001/jamaneurol.2019.3656. PMID: 31657826. PMCID: PMC6820046.
Article23. Kim SM, Woodhall MR, Kim JS, Kim SJ, Park KS, Vincent A, et al. 2015; Antibodies to MOG in adults with inflammatory demyelinating disease of the CNS. Neurol Neuroimmunol Neuroinflamm. 2:e163. DOI: 10.1212/NXI.0000000000000163. PMID: 26516628. PMCID: PMC4608758.
Article24. Lee HL, Kim SH, Seok JM, Kim BJ, Kim HJ, Kim BJ. 2022; Results of a survey on diagnostic procedures and treatment choices for neuromyelitis optica spectrum disorder in Korea: beyond the context of current clinical guidelines. J Clin Neurol. 18:207–13. DOI: 10.3988/jcn.2022.18.2.207. PMID: 35274837. PMCID: PMC8926765.
Article25. Gastaldi M, Scaranzin S, Jarius S, Wildeman B, Zardini E, Mallucci G, et al. 2020; Cell-based assays for the detection of MOG antibodies: a comparative study. J Neurol. 267:3555–64. DOI: 10.1007/s00415-020-10024-0. PMID: 32623596.
Article26. Reindl M, Schanda K, Woodhall M, Tea F, Ramanathan S, Sagen J, et al. 2020; International multicenter examination of MOG antibody assays. Neurol Neuroimmunol Neuroinflamm. 7:e674. DOI: 10.1212/NXI.0000000000000674. PMID: 32024795. PMCID: PMC7051197.
Article27. Lui A, Chong J, Flanagan E, Abrams AW, Krysko KM, Arikan B, et al. 2021; High titers of myelin oligodendrocyte glycoprotein antibody are only observed close to clinical events in pediatrics. Mult Scler Relat Disord. 56:103253. DOI: 10.1016/j.msard.2021.103253. PMID: 34517190. PMCID: PMC8678350.28. Cobo-Calvo A, Sepúlveda M, d'Indy H, Armangué T, Ruiz A, Maillart E, et al. 2019; Usefulness of MOG-antibody titres at first episode to predict the future clinical course in adults. J Neurol. 266:806–15. DOI: 10.1007/s00415-018-9160-9. PMID: 30607536.
Article29. van Pelt ED, Wong YYM, Ketelslegers IA, Hamann D, Hintzen RQ. 2016; Neuromyelitis optica spectrum disorders: comparison of clinical and magnetic resonance imaging characteristics of AQP4-IgG versus MOG-IgG seropositive cases in the Netherlands. Eur J Neurol. 23:580–7. DOI: 10.1111/ene.12898. PMID: 26593750.
Article30. Hyun JW, Kim Y, Kim KH, Kim SH, Olesen MN, Asgari N, et al. 2022; CSF GFAP levels in double seronegative neuromyelitis optica spectrum disorder: no evidence of astrocyte damage. J Neuroinflammation. 19:86. DOI: 10.1186/s12974-022-02450-w. PMID: 35413922. PMCID: PMC9006458.
Article31. Sepúlveda M, Armangué T, Sola-Valls N, Arrambide G, Meca-Lallana JE, Oreja-Guevara C, et al. 2016; Neuromyelitis optica spectrum disorders: comparison according to the phenotype and serostatus. Neurol Neuroimmunol Neuroinflamm. 3:e225. DOI: 10.1212/NXI.0000000000000225. PMID: 27144216. PMCID: PMC4841645.32. Banwell B, Bennett JL, Marignier R, Kim HJ, Brilot F, Flanagan EP, et al. 2023; Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol. 22:268–82. DOI: 10.1016/S1474-4422(22)00431-8. PMID: 36706773.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Myelin oligodendrocyte glycoprotein antibody-associated disorders: clinical spectrum, diagnostic evaluation, and treatment options
- Clinical and Radiological Features of Myelin Oligodendrocyte Glycoprotein-Associated Myelitis in Adults
- Immunocytochemical localization of myelin basic protein, proteolipid protein and myelin-associated glycoprotein in human oligodendrocyte in culture
- Recurrent Optic Neuritis and Acute Encephalopathy with Myelin Oligodendrocyte Glycoprotein Antibodies in a Korean Child
- Anti-Myelin Oligodendrocyte Glycoprotein Syndrome with Findings Resembling “Snake-Eye Appearance”: a Case Report