Ann Rehabil Med.
2023 Dec;47(6):444-458. 10.5535/arm.23131.
AI in Rehabilitation Medicine: Opportunities and Challenges
- Affiliations
-
- 1Max Nader Lab for Rehabilitation Technologies and Outcomes Research, Shirley Ryan AbilityLab, Chicago, IL, United States
- 2Department of Physical Medicine and Rehabilitation, Northwestern University, Chicago, IL, United States
- KMID: 2549726
- DOI: http://doi.org/10.5535/arm.23131
Abstract
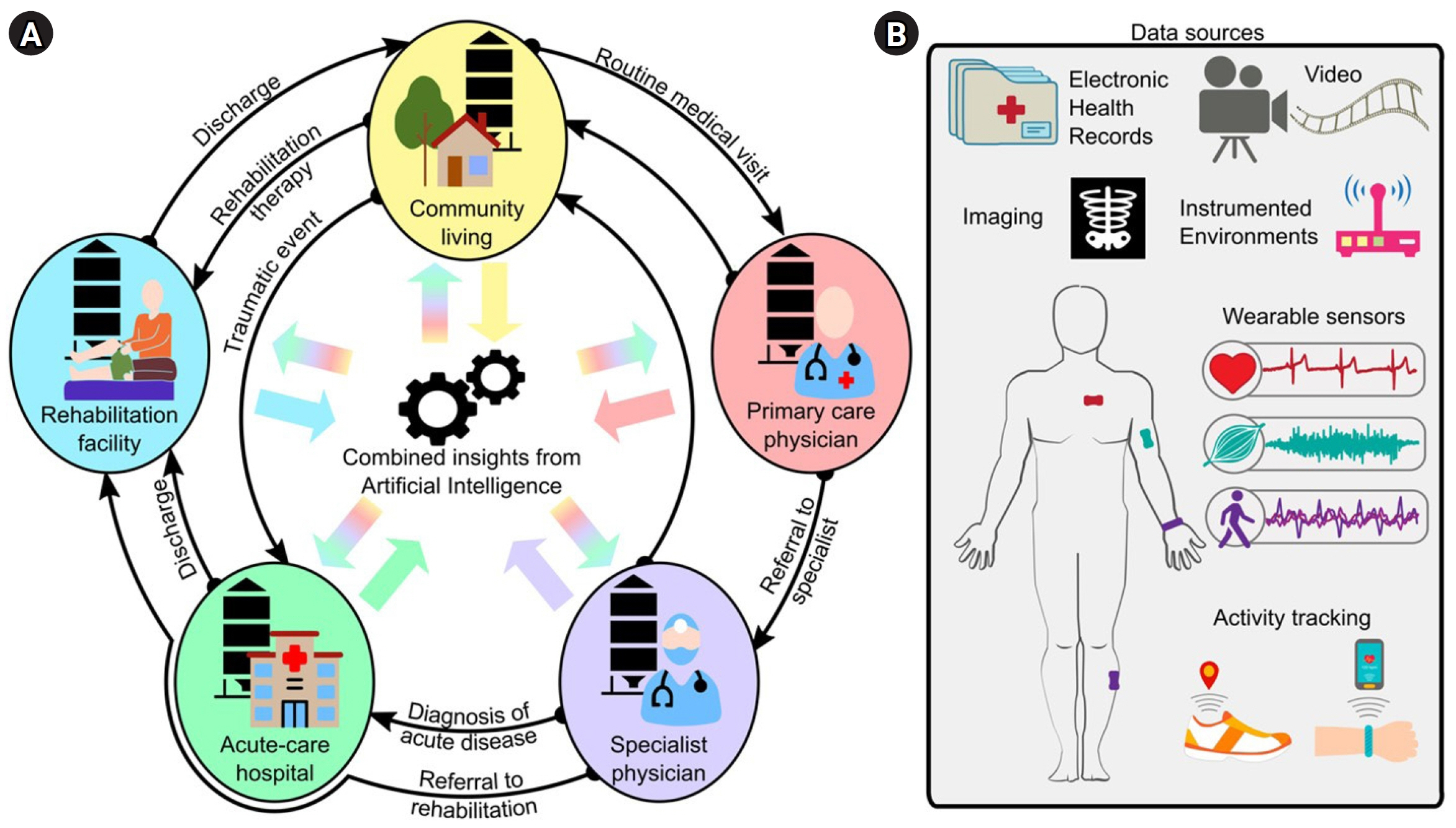
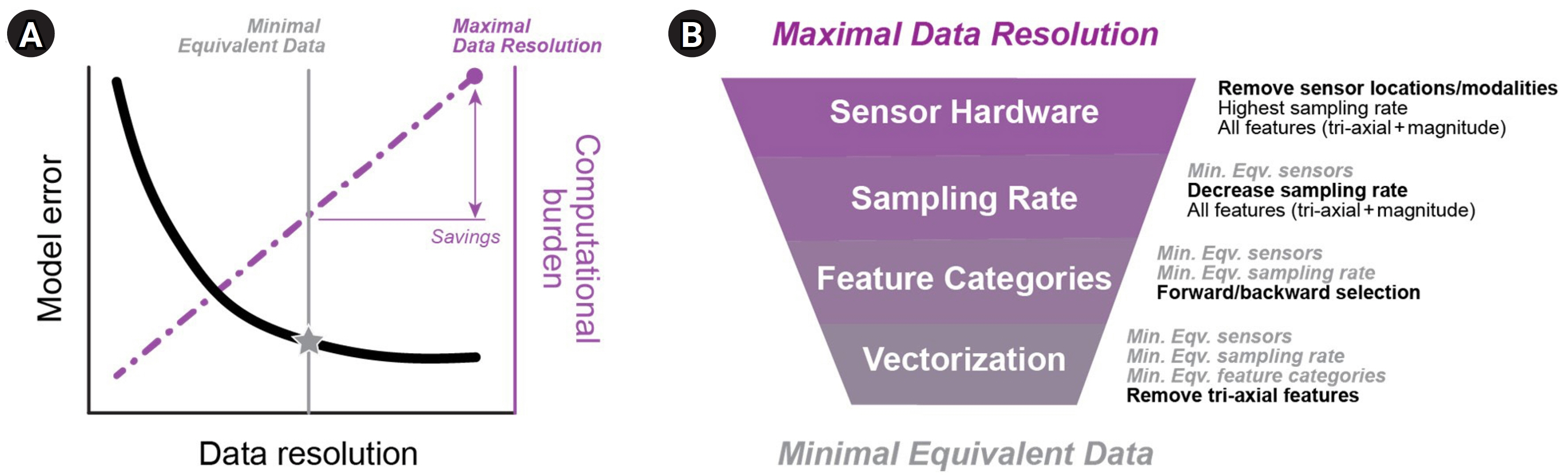
- Artificial intelligence (AI) tools are increasingly able to learn from larger and more complex data, thus allowing clinicians and scientists to gain new insights from the information they collect about their patients every day. In rehabilitation medicine, AI can be used to find patterns in huge amounts of healthcare data. These patterns can then be leveraged at the individual level, to design personalized care strategies and interventions to optimize each patient’s outcomes. However, building effective AI tools requires many careful considerations about how we collect and handle data, how we train the models, and how we interpret results. In this perspective, we discuss some of the current opportunities and challenges for AI in rehabilitation. We first review recent trends in AI for the screening, diagnosis, treatment, and continuous monitoring of disease or injury, with a special focus on the different types of healthcare data used for these applications. We then examine potential barriers to designing and integrating AI into the clinical workflow, and we propose an end-to-end framework to address these barriers and guide the development of effective AI for rehabilitation. Finally, we present ideas for future work to pave the way for AI implementation in real-world rehabilitation practices.
Figure
Reference
-
1. Korteling JEH, van de Boer-Visschedijk GC, Blankendaal RAM, Boonekamp RC, Eikelboom AR. Human- versus Artificial Intelligence. Front Artif Intell. 2021; 4:622364.
Article2. Yu KH, Beam AL, Kohane IS. Artificial intelligence in healthcare. Nat Biomed Eng. 2018; 2:719–31.
Article3. van der Schaar M, Zame W. Machine learning for individualised medicine. In : Pearson-Stuttard J, Murphy O, editors. Annual report of the chief medical officer, 2018. Health 2040 – better health within reach. Department of Health and Social Care;2018.4. French MA, Roemmich RT, Daley K, Beier M, Penttinen S, Raghavan P, et al. Precision rehabilitation: optimizing function, adding value to health care. Arch Phys Med Rehabil. 2022; 103:1233–9.
Article5. Agrawal R, Prabakaran S. Big data in digital healthcare: lessons learnt and recommendations for general practice. Heredity (Edinb). 2020; 124:525–34.
Article6. Reda R, Piccinini F, Carbonaro A. Towards consistent data representation in the IoT healthcare landscape. Paper presented at: DH '18: Proceedings of the 2018 International Conference on Digital Health; 2018 Apr 23-26; Lyon, France. p. 5-10.
Article7. Bellazzi R, Zupan B. Predictive data mining in clinical medicine: current issues and guidelines. Int J Med Inform. 2008; 77:81–97.
Article8. Deo RC. Machine learning in medicine. Circulation. 2015; 132:1920–30.
Article9. Landi I, Glicksberg BS, Lee HC, Cherng S, Landi G, Danieletto M, et al. Deep representation learning of electronic health records to unlock patient stratification at scale. NPJ Digit Med. 2020; 3:96.
Article10. Jensen PB, Jensen LJ, Brunak S. Mining electronic health records: towards better research applications and clinical care. Nat Rev Genet. 2012; 13:395–405.
Article11. Xu S, Kim J, Walter JR, Ghaffari R, Rogers JA. Translational gaps and opportunities for medical wearables in digital health. Sci Transl Med. 2022; 14:eabn6036.
Article12. Brom H, Brooks Carthon JM, Ikeaba U, Chittams J. Leveraging electronic health records and machine learning to tailor nursing care for patients at high risk for readmissions. J Nurs Care Qual. 2020; 35:27–33.
Article13. Delahanty RJ, Alvarez J, Flynn LM, Sherwin RL, Jones SS. Development and evaluation of a machine learning model for the early identification of patients at risk for sepsis. Ann Emerg Med. 2019; 73:334–44.
Article14. Engelhard MM, Henao R, Berchuck SI, Chen J, Eichner B, Herkert D, et al. Predictive value of early autism detection models based on electronic health record data collected before age 1 year. JAMA Netw Open. 2023; 6:e2254303.
Article15. Flores AM, Demsas F, Leeper NJ, Ross EG. Leveraging machine learning and artificial intelligence to improve peripheral artery disease detection, treatment, and outcomes. Circ Res. 2021; 128:1833–50.16. Ye C, Li J, Hao S, Liu M, Jin H, Zheng L, et al. Identification of elders at higher risk for fall with statewide electronic health records and a machine learning algorithm. Int J Med Inform. 2020; 137:104105.
Article17. Cramer EM, Seneviratne MG, Sharifi H, Ozturk A, Hernandez-Boussard T. Predicting the incidence of pressure ulcers in the intensive care unit using machine learning. EGEMS (Wash DC). 2019; 7:49.
Article18. Kucukboyaci NE, Long C, Smith M, Rath JF, Bushnik T. Cluster analysis of vulnerable groups in acute traumatic brain injury rehabilitation. Arch Phys Med Rehabil. 2018; 99:2365–9.
Article19. Custer MG, Huebner RA. Identifying homogeneous outcome groups in adult rehabilitation using cluster analysis. Am J Occup Ther. 2019; 73:7305205050p1–9.
Article20. Bland MD, Sturmoski A, Whitson M, Connor LT, Fucetola R, Huskey T, et al. Prediction of discharge walking ability from initial assessment in a stroke inpatient rehabilitation facility population. Arch Phys Med Rehabil. 2012; 93:1441–7.
Article21. Harari Y, O'Brien MK, Lieber RL, Jayaraman A. Inpatient stroke rehabilitation: prediction of clinical outcomes using a machine-learning approach. J Neuroeng Rehabil. 2020; 17:71.
Article22. Henderson CE, Fahey M, Brazg G, Moore JL, Hornby TG. Predicting discharge walking function with high-intensity stepping training during inpatient rehabilitation in nonambulatory patients poststroke. Arch Phys Med Rehabil. 2022; 103(7S):S189–96.
Article23. Scrutinio D, Lanzillo B, Guida P, Mastropasqua F, Monitillo V, Pusineri M, et al. Development and validation of a predictive model for functional outcome after stroke rehabilitation: the Maugeri model. Stroke. 2017; 48:3308–15.
Article24. Stinear CM, Smith MC, Byblow WD. Prediction tools for stroke rehabilitation. Stroke. 2019; 50:3314–22.
Article25. Smith MC, Barber AP, Scrivener BJ, Stinear CM. The TWIST tool predicts when patients will recover independent walking after stroke: an observational study. Neurorehabil Neural Repair. 2022; 36:461–71.
Article26. Lundquist CB, Nielsen JF, Arguissain FG, Brunner IC. Accuracy of the upper limb prediction algorithm PREP2 applied 2 weeks poststroke: a prospective longitudinal study. Neurorehabil Neural Repair. 2021; 35:68–78.
Article27. Ehteshami Bejnordi B, Veta M, Johannes van Diest P, van Ginneken B, Karssemeijer N, Litjens G, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA. 2017; 318:2199–210.
Article28. Litjens G, Sánchez CI, Timofeeva N, Hermsen M, Nagtegaal I, Kovacs I, et al. Deep learning as a tool for increased accuracy and efficiency of histopathological diagnosis. Sci Rep. 2016; 6:26286.
Article29. Poplin R, Varadarajan AV, Blumer K, Liu Y, McConnell MV, Corrado GS, et al. Prediction of cardiovascular risk factors from retinal fundus photographs via deep learning. Nat Biomed Eng. 2018; 2:158–64.
Article30. Panwar N, Huang P, Lee J, Keane PA, Chuan TS, Richhariya A, et al. Fundus photography in the 21st century--a review of recent technological advances and their implications for worldwide healthcare. Telemed J E Health. 2016; 22:198–208.
Article31. Solana-Lavalle G, Rosas-Romero R. Classification of PPMI MRI scans with voxel-based morphometry and machine learning to assist in the diagnosis of Parkinson's disease. Comput Methods Programs Biomed. 2021; 198:105793.
Article32. Liew SL, Schweighofer N, Cole JH, Zavaliangos-Petropulu A, Lo BP, Han LKM, et al. Association of brain age, lesion volume, and functional outcome in patients with stroke. Neurology. 2023; 100:e2103–13.33. Warmerdam E, Hausdorff JM, Atrsaei A, Zhou Y, Mirelman A, Aminian K, et al. Long-term unsupervised mobility assessment in movement disorders. Lancet Neurol. 2020; 19:462–70.
Article34. Adans-Dester CP, Lang CE, Reinkensmeyer DJ, Bonato P. Wearable sensors for stroke rehabilitation. In : Reinkensmeyer DJ, Marchal-Crespo L, Dietz V, editors. Neurorehabilitation technology. Springer;2022. p. 467–507.35. O'Brien MK, Shin SY, Khazanchi R, Fanton M, Lieber RL, Ghaffari R, et al. Wearable sensors improve prediction of post-stroke walking function following inpatient rehabilitation. IEEE J Transl Eng Health Med. 2022; 10:2100711.36. Liuzzi P, Carpinella I, Anastasi D, Gervasoni E, Lencioni T, Bertoni R, et al. Machine learning based estimation of dynamic balance and gait adaptability in persons with neurological diseases using inertial sensors. Sci Rep. 2023; 13:8640.
Article37. Adans-Dester C, Hankov N, O'Brien A, Vergara-Diaz G, Black-Schaffer R, Zafonte R, et al. Enabling precision rehabilitation interventions using wearable sensors and machine learning to track motor recovery. NPJ Digit Med. 2020; 3:121.
Article38. Lonini L, Dai A, Shawen N, Simuni T, Poon C, Shimanovich L, et al. Wearable sensors for Parkinson's disease: which data are worth collecting for training symptom detection models. NPJ Digit Med. 2018; 1:64.
Article39. Shawen N, O'Brien MK, Venkatesan S, Lonini L, Simuni T, Hamilton JL, et al. Role of data measurement characteristics in the accurate detection of Parkinson's disease symptoms using wearable sensors. J Neuroeng Rehabil. 2020; 17:52.
Article40. O'Brien MK, Botonis OK, Larkin E, Carpenter J, Martin-Harris B, Maronati R, et al. Advanced machine learning tools to monitor biomarkers of dysphagia: a wearable sensor proof-of-concept study. Digit Biomark. 2021; 5:167–75.41. Hafer JF, Vitali R, Gurchiek R, Curtze C, Shull P, Cain SM. Challenges and advances in the use of wearable sensors for lower extremity biomechanics. J Biomech. 2023; 157:111714.
Article42. Albert MV, Deeny S, McCarthy C, Valentin J, Jayaraman A. Monitoring daily function in persons with transfemoral amputations using a commercial activity monitor: a feasibility study. PM R. 2014; 6:1120–7.
Article43. Kim J, Colabianchi N, Wensman J, Gates DH. Wearable sensors quantify mobility in people with lower limb amputation during daily life. IEEE Trans Neural Syst Rehabil Eng. 2020; 28:1282–91.
Article44. Moshawrab M, Adda M, Bouzouane A, Ibrahim H, Raad A. Smart wearables for the detection of cardiovascular diseases: a systematic literature review. Sensors (Basel). 2023; 23:828.
Article45. Boe AJ, McGee Koch LL, O'Brien MK, Shawen N, Rogers JA, Lieber RL, et al. Automating sleep stage classification using wireless, wearable sensors. NPJ Digit Med. 2019; 2:131.
Article46. Hsieh KL, Chen L, Sosnoff JJ. Mobile technology for falls prevention in older adults. J Gerontol A Biol Sci Med Sci. 2023; 78:861–8.47. Ghomrawi HMK, O'Brien MK, Carter M, Macaluso R, Khazanchi R, Fanton M, et al. Applying machine learning to consumer wearable data for the early detection of complications after pediatric appendectomy. NPJ Digit Med. 2023; 6:148.
Article48. O'Brien MK, Shawen N, Mummidisetty CK, Kaur S, Bo X, Poellabauer C, et al. Activity recognition for persons with stroke using mobile phone technology: toward improved performance in a home setting. J Med Internet Res. 2017; 19:e184.49. Adib F, Mao H, Kabelac Z, Katabi D, Miller RC. Smart homes that monitor breathing and heart rate. Paper presented at: CHI '15: Proceedings of the 33rd Annual ACM Conference on Human Factors in Computing Systems; 2015 Apr 18-23; Seoul, Korea. p. 837-46.
Article50. Zhang G, Vahia IV, Liu Y, Yang Y, May R, Cray HV, et al. Contactless in-home monitoring of the long-term respiratory and behavioral phenotypes in older adults with COVID-19: a case series. Front Psychiatry. 2021; 12:754169.
Article51. Yang Y, Yuan Y, Zhang G, Wang H, Chen YC, Liu Y, et al. Artificial intelligence-enabled detection and assessment of Parkinson's disease using nocturnal breathing signals. Nat Med. 2022; 28:2207–15.
Article52. Haque A, Milstein A, Fei-Fei L. Illuminating the dark spaces of healthcare with ambient intelligence. Nature. 2020; 585:193–202.
Article53. Kim WS, Cho S, Baek D, Bang H, Paik NJ. Upper extremity functional evaluation by Fugl-Meyer Assessment scoring using depth-sensing camera in hemiplegic stroke patients. PLoS One. 2016; 11:e0158640.
Article54. Stenum J, Rossi C, Roemmich RT. Two-dimensional video-based analysis of human gait using pose estimation. PLoS Comput Biol. 2021; 17:e1008935.
Article55. Sato K, Nagashima Y, Mano T, Iwata A, Toda T. Quantifying normal and parkinsonian gait features from home movies: practical application of a deep learning-based 2D pose estimator. PLoS One. 2019; 14:e0223549.
Article56. Lonini L, Moon Y, Embry K, Cotton RJ, McKenzie K, Jenz S, et al. Video-based pose estimation for gait analysis in stroke survivors during clinical assessments: a proof-of-concept study. Digit Biomark. 2022; 6:9–18.57. Lam WWT, Tang YM, Fong KNK. A systematic review of the applications of markerless motion capture (MMC) technology for clinical measurement in rehabilitation. J Neuroeng Rehabil. 2023; 20:57.
Article58. Sibley KG, Girges C, Hoque E, Foltynie T. Video-based analyses of Parkinson's disease severity: a brief review. J Parkinsons Dis. 2021; 11(S1):S83–93.
Article59. Adde L, Helbostad JL, Jensenius AR, Taraldsen G, Grunewaldt KH, Støen R. Early prediction of cerebral palsy by computer-based video analysis of general movements: a feasibility study. Dev Med Child Neurol. 2010; 52:773–8.
Article60. Chen PC, Liu Y, Peng L. How to develop machine learning models for healthcare. Nat Mater. 2019; 18:410–4.
Article61. Alaa AM, van der Schaar M. Demystifying black-box models with symbolic metamodels. Paper presented at: Advances in Neural Information Processing Systems 32 (NeurIPS 2019); 2019 Dec 8-14; Vancouver, Canada. p. 32.62. Ehrmann DE, Joshi S, Goodfellow SD, Mazwi ML, Eytan D. Making machine learning matter to clinicians: model actionability in medical decision-making. NPJ Digit Med. 2023; 6:7.
Article63. Parimbelli E, Buonocore TM, Nicora G, Michalowski W, Wilk S, Bellazzi R. Why did AI get this one wrong? - tree-based explanations of machine learning model predictions. Artif Intell Med. 2023; 135:102471.
Article64. Busch Tde A, Duarte YA, Pires Nunes D, Lebrão ML, Satya Naslavsky M, dos Santos Rodrigues A, et al. Factors associated with lower gait speed among the elderly living in a developing country: a cross-sectional population-based study. BMC Geriatr. 2015; 15:35.
Article65. Pacini Panebianco G, Bisi MC, Stagni R, Fantozzi S. Analysis of the performance of 17 algorithms from a systematic review: influence of sensor position, analysed variable and computational approach in gait timing estimation from IMU measurements. Gait Posture. 2018; 66:76–82.
Article66. Atallah L, Lo B, King R, Yang GZ. Sensor positioning for activity recognition using wearable accelerometers. IEEE Trans Biomed Circuits Syst. 2011; 5:320–9.
Article67. Rantz M, Phillips LJ, Galambos C, Lane K, Alexander GL, Despins L, et al. Randomized trial of intelligent sensor system for early illness alerts in senior housing. J Am Med Dir Assoc. 2017; 18:860–70.
Article68. Luo Z, Hsieh JT, Balachandar N, Yeung S, Pusiol G, Luxenberg J, et al. Computer vision-based descriptive analytics of seniors’ daily activities for long-term health monitoring. Proc Mach Learn Res. 2018; 85:1–18.69. Gold R, Reichman M, Greenberg E, Ivanidze J, Elias E, Tsiouris AJ, et al. Developing a new reference standard: is validation necessary? Acad Radiol. 2010; 17:1079–82.70. Fusca M, Negrini F, Perego P, Magoni L, Molteni F, Andreoni G. Validation of a wearable IMU system for gait analysis: protocol and application to a new system. Appl Sci. 2018; 8:1167.
Article71. van Lier HG, Pieterse ME, Garde A, Postel MG, de Haan HA, Vollenbroek-Hutten MMR, et al. A standardized validity assessment protocol for physiological signals from wearable technology: methodological underpinnings and an application to the E4 biosensor. Behav Res Methods. 2020; 52:607–29.
Article72. Lonini L, Gupta A, Deems-Dluhy S, Hoppe-Ludwig S, Kording K, Jayaraman A. Activity recognition in individuals walking with assistive devices: the benefits of device-specific models. JMIR Rehabil Assist Technol. 2017; 4:e8.
Article73. Lanotte F, Shin SY, O'Brien MK, Jayaraman A. Validity and reliability of a commercial wearable sensor system for measuring spatiotemporal gait parameters in a post-stroke population: the effects of walking speed and asymmetry. Physiol Meas. 2023; 44:085005.
Article74. Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci. 2010; 1186:69–101.
Article75. Prates MOR, Avelar PHC, Lamb L. Assessing gender bias in machine translation: a case study with Google Translate. Neural Comput Appl. 2020; 32:6363–81.
Article76. Kohane IS, Aronow BJ, Avillach P, Beaulieu-Jones BK, Bellazzi R, Bradford RL, et al. What every reader should know about studies using electronic health record data but may be afraid to ask. J Med Internet Res. 2021; 23:e22219.
Article77. Zijlstra W, Hof AL. Assessment of spatio-temporal gait parameters from trunk accelerations during human walking. Gait Posture. 2003; 18:1–10.
Article78. Jasiewicz JM, Allum JH, Middleton JW, Barriskill A, Condie P, Purcell B, et al. Gait event detection using linear accelerometers or angular velocity transducers in able-bodied and spinal-cord injured individuals. Gait Posture. 2006; 24:502–9.
Article79. Nazmi N, Abdul Rahman MA, Yamamoto S, Ahmad SA, Zamzuri H, Mazlan SA. A review of classification techniques of EMG signals during isotonic and isometric contractions. Sensors (Basel). 2016; 16:1304.
Article80. Herrero JG, Patricio MA, Molina JM, Cardoso LA. Contextual and human factors in information fusion. IOS Press Ebooks;2010. p. 79–92.81. Yu L, Zhou R, Chen R, Lai KK. Missing data preprocessing in credit classification: one-hot encoding or imputation? Emerg Markets Financ Trade. 2020; 58:472–82.
Article82. Guyon I, Elisseef A. An introduction to variable and feature selection. J Mach Learn Res. 2003; 3:1157–82.83. Baxter J. A model of inductive bias learning. J Artif Intell Res. 2000; 12:149–98.
Article84. Vranas KC, Jopling JK, Sweeney TE, Ramsey MC, Milstein AS, Slatore CG, et al. Identifying distinct subgroups of ICU patients: a machine learning approach. Crit Care Med. 2017; 45:1607–15.
Article85. Turgeman L, May JH, Sciulli R. Insights from a machine learning model for predicting the hospital Length of Stay (LOS) at the time of admission. Expert Syst Appl. 2017; 78:376–85.
Article86. Lonini L, Shawen N, Ghaffari R, Rogers J, Jayarman A. Automatic detection of spasticity from flexible wearable sensors. Paper presented at: UbiComp '17: Proceedings of the 2017 ACM International Joint Conference on Pervasive and Ubiquitous Computing and Proceedings of the 2017 ACM International Symposium on Wearable Computers; 2017 Sep 11-15; Maui, Hawaii. p. 133-6.
Article87. Lonini L, Shawen N, Botonis O, Fanton M, Jayaraman C, Mummidisetty CK, et al. Rapid screening of physiological changes associated with COVID-19 using soft-wearables and structured activities: a pilot study. IEEE J Transl Eng Health Med. 2021; 9:4900311.
Article88. Bacciu D, Chessa S, Gallicchio C, Micheli A, Pedrelli L, Ferro E, et al. A learning system for automatic Berg Balance Scale score estimation. Eng Appl Artif Intell. 2017; 66:60–74.
Article89. Krishnan R, Rajpurkar P, Topol EJ. Self-supervised learning in medicine and healthcare. Nat Biomed Eng. 2022; 6:1346–52.
Article90. Yoon J, Drumright LN, van der Schaar M. Anonymization through data synthesis using generative adversarial networks (ADS-GAN). IEEE J Biomed Health Inform. 2020; 24:2378–88.
Article91. Yang X, Chen A, PourNejatian N, Shin HC, Smith KE, Parisien C, et al. A large language model for electronic health records. NPJ Digit Med. 2022; 5:194.
Article92. Saeb S, Lonini L, Jayaraman A, Mohr DC, Kording KP. The need to approximate the use-case in clinical machine learning. Gigascience. 2017; 6:1–9.
Article93. Ramspek CL, Jager KJ, Dekker FW, Zoccali C, van Diepen M. External validation of prognostic models: what, why, how, when and where? Clin Kidney J. 2020; 14:49–58.94. Gautam A, Panwar M, Biswas D, Acharyya A. MyoNet: a transfer-learning-based LRCN for lower limb movement recognition and knee joint angle prediction for remote monitoring of rehabilitation progress from sEMG. IEEE J Transl Eng Health Med. 2020; 8:2100310.
Article95. Du C, Graham S, Depp C, Nguyen T. Assessing physical rehabilitation exercises using graph convolutional network with self-supervised regularization. Annu Int Conf IEEE Eng Med Biol Soc. 2021; 2021:281–5.
Article96. Chen RJ, Lu MY, Chen TY, Williamson DFK, Mahmood F. Synthetic data in machine learning for medicine and healthcare. Nat Biomed Eng. 2021; 5:493–7.
Article97. Che Z, Cheng Y, Zhai S, Sun Z, Liu Y. Boosting deep learning risk prediction with generative adversarial networks for electronic health records. Paper presented at: 2017 IEEE International Conference on Data Mining (ICDM); 2017 Nov 18-21; New Orleans, USA. p. 787-92.
Article98. Shen C, Wang Z, Villar SS, Van Der Schaar M. Learning for dose allocation in adaptive clinical trials with safety constraints. Paper presented at: ICML'20: Proceedings of the 37th International Conference on Machine Learning; 2020 Jul 13-18; Virtual Event. p. 8730-40.99. Arora A, Arora A. The promise of large language models in health care. Lancet. 2023; 401:641.
Article100. Murphy C, Thomas FP. Generative AI in spinal cord injury research and care: opportunities and challenges ahead. J Spinal Cord Med. 2023; 46:341–2.
Article101. Thirunavukarasu AJ. Large language models will not replace healthcare professionals: curbing popular fears and hype. J R Soc Med. 2023; 116:181–2.
Article102. Singhal K, Azizi S, Tu T, Mahdavi SS, Wei J, Chung HW, et al. Large language models encode clinical knowledge. Nature. 2023; 620:172–80. Erratum in: Nature 2023;620:E19.
Article103. Vasey B, Nagendran M, Campbell B, Clifton DA, Collins GS, Denaxas S, et al. Reporting guideline for the early-stage clinical evaluation of decision support systems driven by artificial intelligence: DECIDE-AI. Nat Med. 2022; 28:924–33. Erratum in: Nat Med 2022;28:2218.104. Sahiner B, Chen W, Samala RK, Petrick N. Data drift in medical machine learning: implications and potential remedies. Br J Radiol. 2023; 96:20220878.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Situation of Cancer Treatment in Ethiopia: Challenges and Opportunities
- Challenges and Opportunities toward the Decade of Healthy Ageing in the Post-pandemic Era
- Recent Advances in the Application of Artificial Intelligence in Otorhinolaryngology-Head and Neck Surgery
- Explainable artificial intelligence in emergency medicine: an overview
- Artificial Intelligence in Aviation